FAQ
FAQ
-
According to ISO Guide 30, an RMP is a (public or private) organization or company that is fully responsible for:
- Project planning and manufacturing
- Assignment of property values and relevant uncertainties
- Decision on property values and relevant uncertainties
- Authorization of property values
- Issuance of a reference material certificate or other statements for the reference materials it produces.
[SOURCE: ISO Guide 30:2015, 2.3.5]
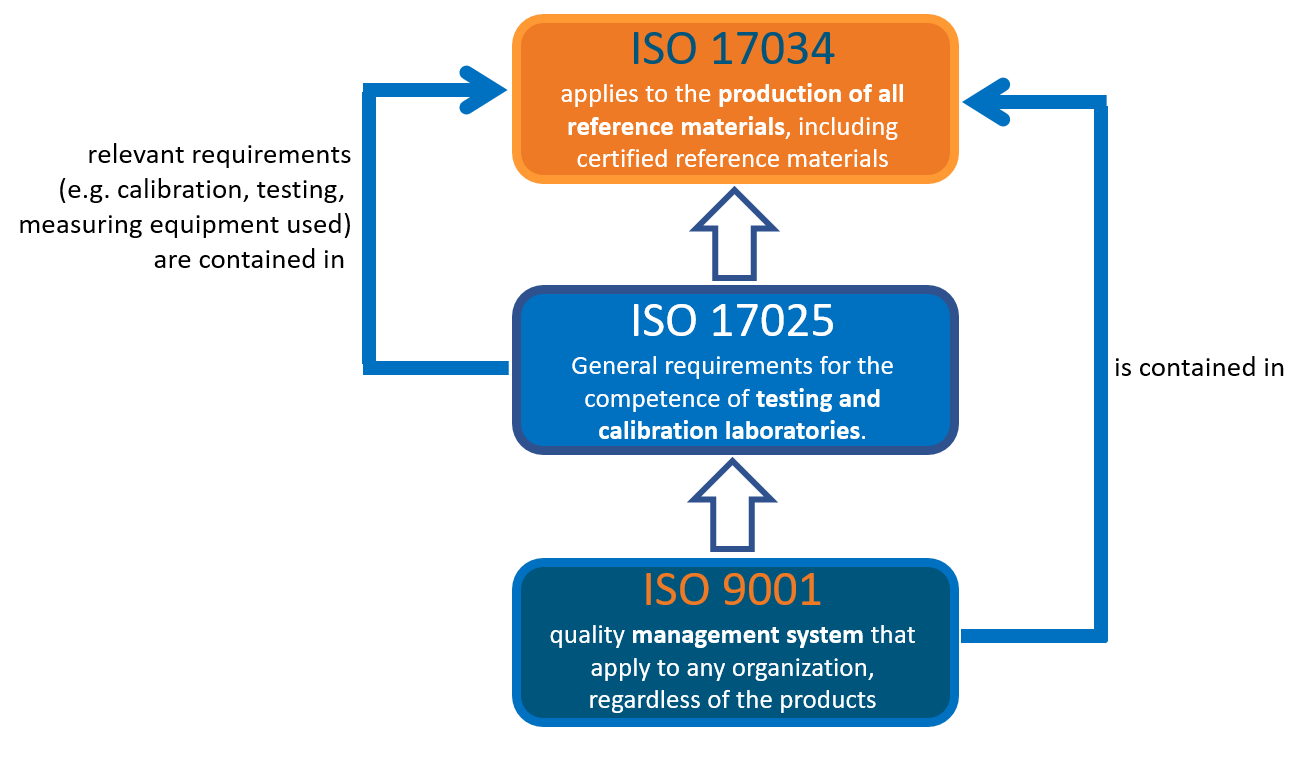
The International Organization for Standardization (ISO) has developed several standardized quality assurance procedures for product manufacturers. These standards ensure that users and customers receive high-quality and reliable products. Among many others, the three most important norms are ISO 9001, ISO 17025 and ISO 17034.
- ISO 17025 is the standard for General Requirements for the Competence of Testing and Calibration Laboratories.
- ISO 17034 is the fundamental standard for reference material manufacturers. It applies to the manufacture of all reference materials, including certified reference materials, and defines the requirements that must be met in the competent manufacture of high-quality and certified reference materials.
- This standard refers to the production of reference materials used in the measurement process, including validation, calibration and quality control.
- ISO 17034 is in accordance with the relevant requirements of ISO 17025 and requires that:
- the relevant requirements according to ISO 17025 regarding calibration and testing are fulfilled
- the measuring equipment used in the manufacture of RM is used in accordance with the applicable requirements of ISO 17025
- metrological traceability of the certified values is ensured during the manufacture of CRMs in accordance with the relevant requirements of ISO 17025.
- In contrast to ISO 17025, ISO 17034 additionally requires reliable and traceable manufacturing of the products.
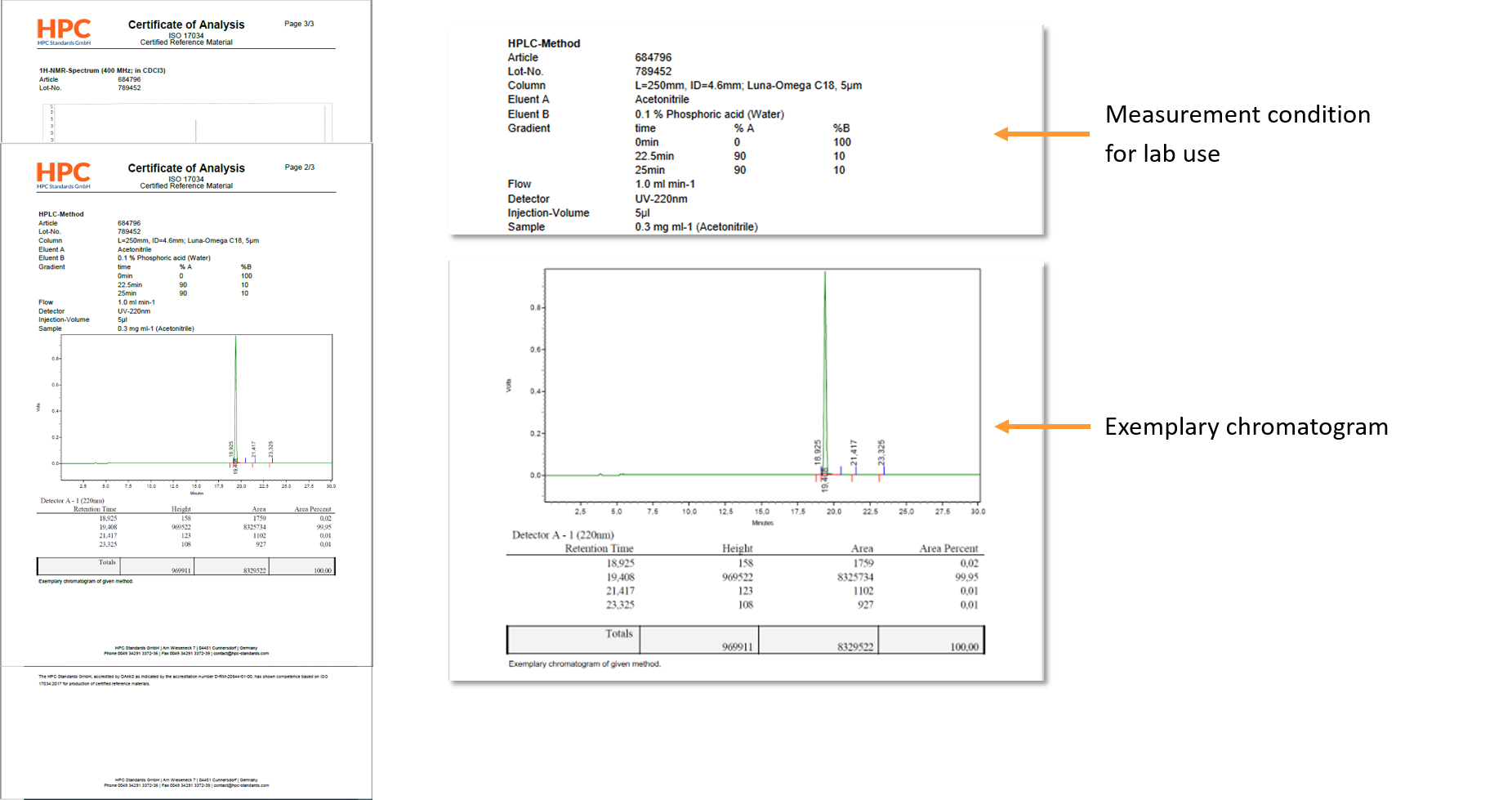
Reference materials are used in all phases of the measurement process, including method validation, calibration and quality control. They are also used in interlaboratory comparisons for method validation and laboratory performance evaluation. A reference material is a material that is sufficiently homogeneous and stable and indicates this on a certificate.
->These data are included in the calculation of the Expanded Uncertainty on the certificates (i.e. Product Information Sheet).
Note 3 to 3.3 of ISO 17034 defines the uses of RMs (calibration of a measurement system, evaluation of a measurement procedure, assignment of values to other materials, quality control). (ISO 17025, 6.4.1, Note 1; as well as 17034, 3.3 and ISO Guide 33). Furthermore, the ISO Guide 33, 1.2 (The guide is intended to give general recommendations for the use of RMs) that RMs where traceability of property values is not given can nevertheless be used to evaluate parts of the measurement procedures and evaluate different levels of precision. (ISO Guide 33, Guide is to provide general recommendations on the use of RMs)
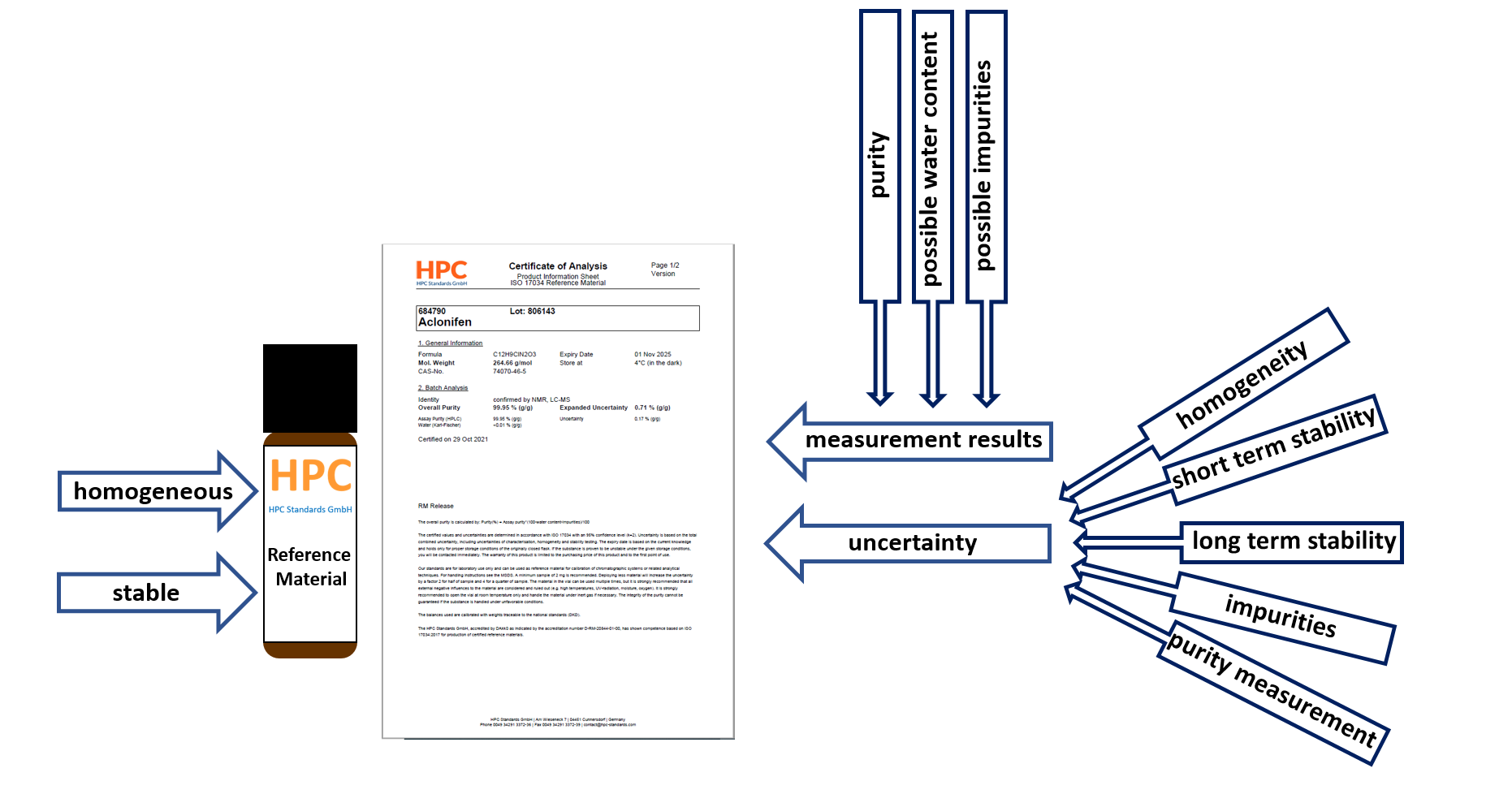
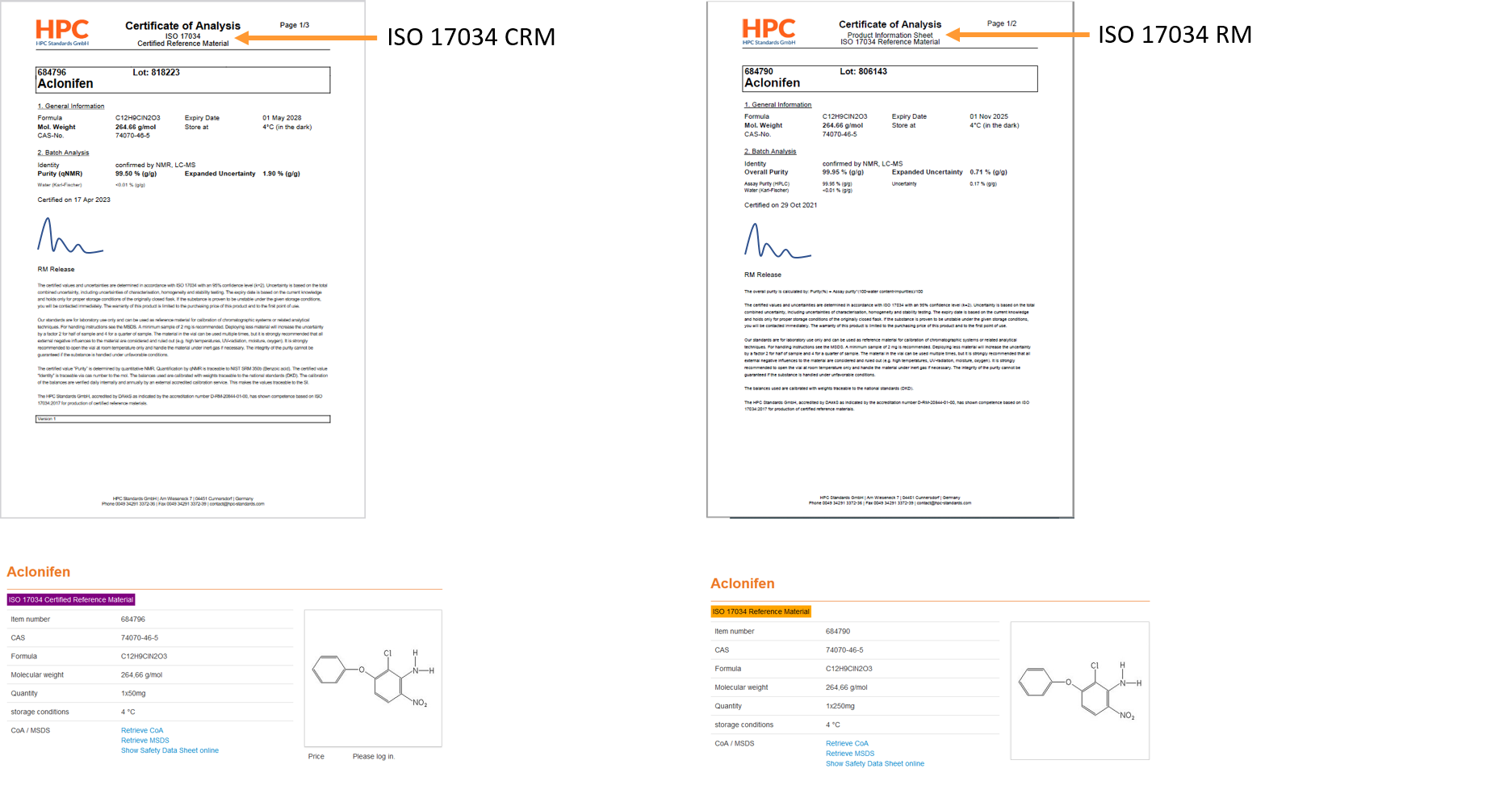
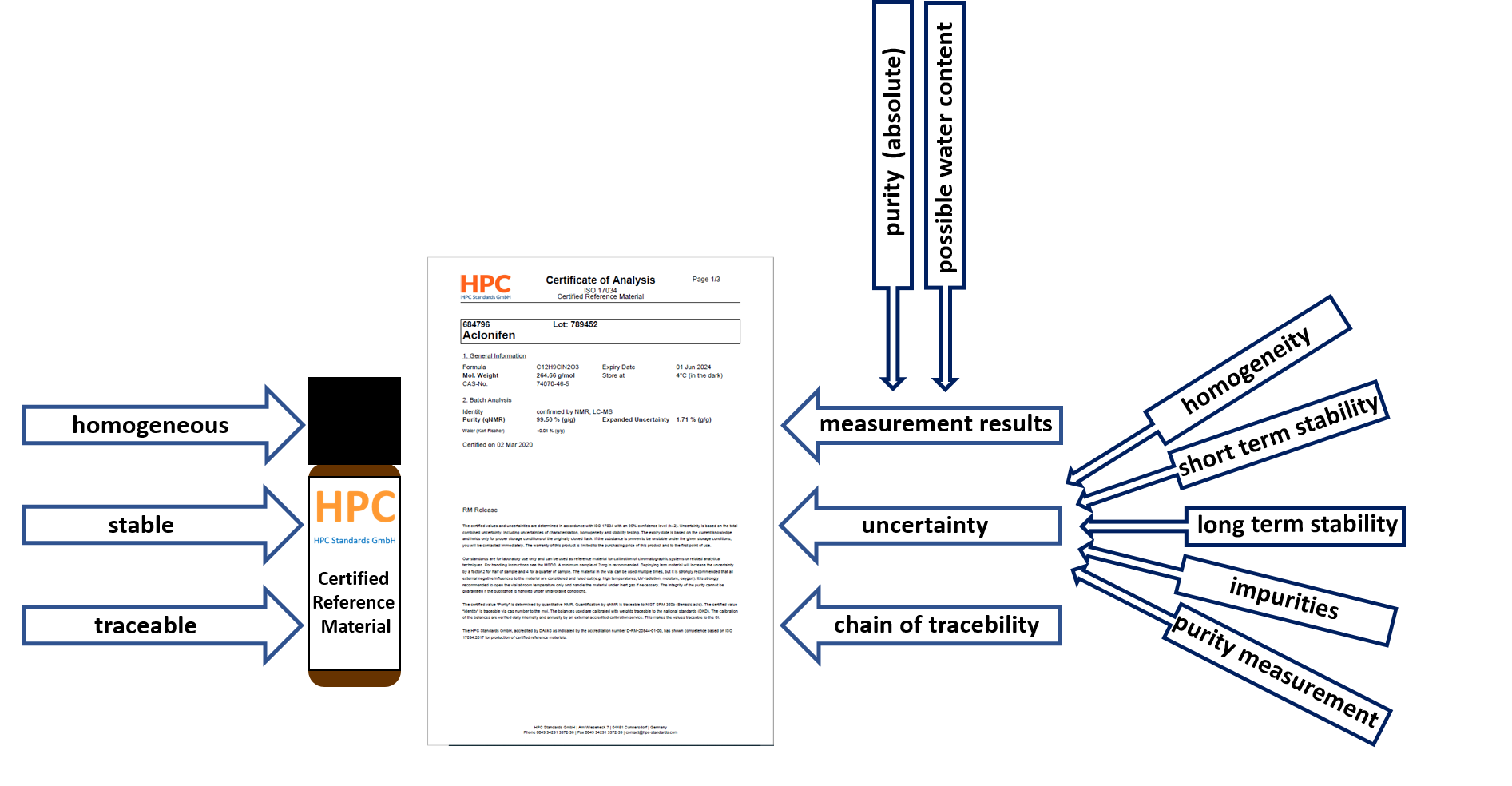
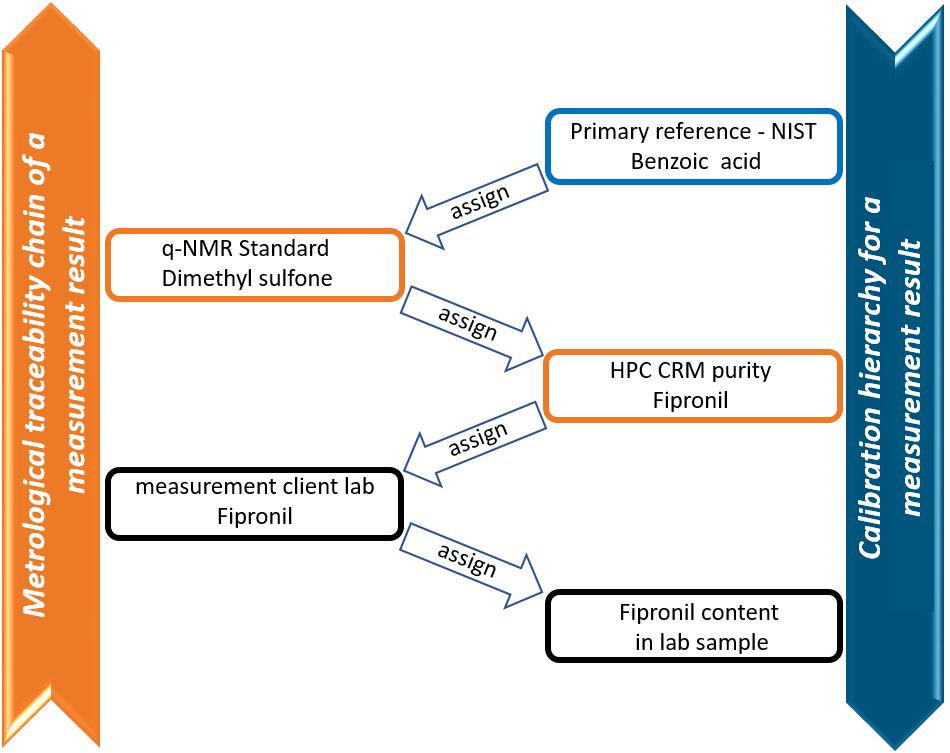
A certified reference material is a material that is sufficiently homogeneous and stable. In addition, the certificate shows certified values, their uncertainty and traceability. For traceability of a certified reference material, there must be an unbroken chain of traceability to a NIST standard (or other primary standard).
-> This data is specified on the Certificate of Analysis.
CRMs are typically used to calibrate measuring instruments and measurement systems.
CRMs can also be used for comparisons that confirm chemical identification or elemental composition
(ISO 17025, 6.4.1, Note 1; as well as 17034, 3.3 and ISO Guide 33)
Unbroken chain of traceability to a NIST standard.
Easy-to-find, ISO-compliant documentation, as all relevant information is included in the COA (i.e. Product information sheet):- Purity
- Confidence interval/ Expanded uncertainty (composed of the uncertainties in characterization, homogeneity and stability testing)
- Minimum sample size
- Handling of the compound (storage conditions, use)
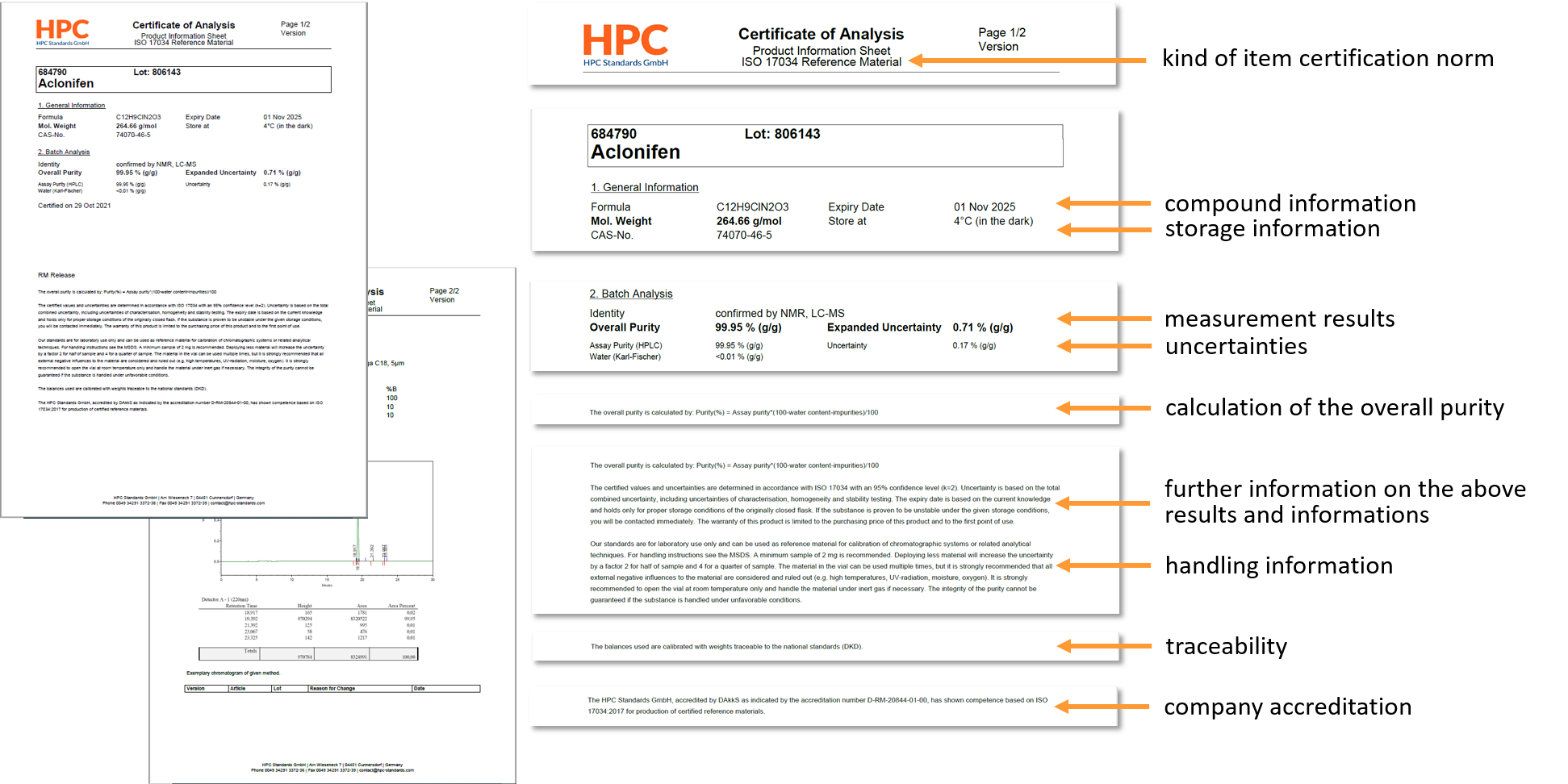
Easy-to-find, ISO-compliant documentation, as all relevant information is included in the COA:- Purity
- Confidence interval/ Expanded uncertainty (composed of the uncertainties in characterization, homogeneity and stability testing)
- Minimum sample size
- Traceability to primary standard (e.g. NIST).
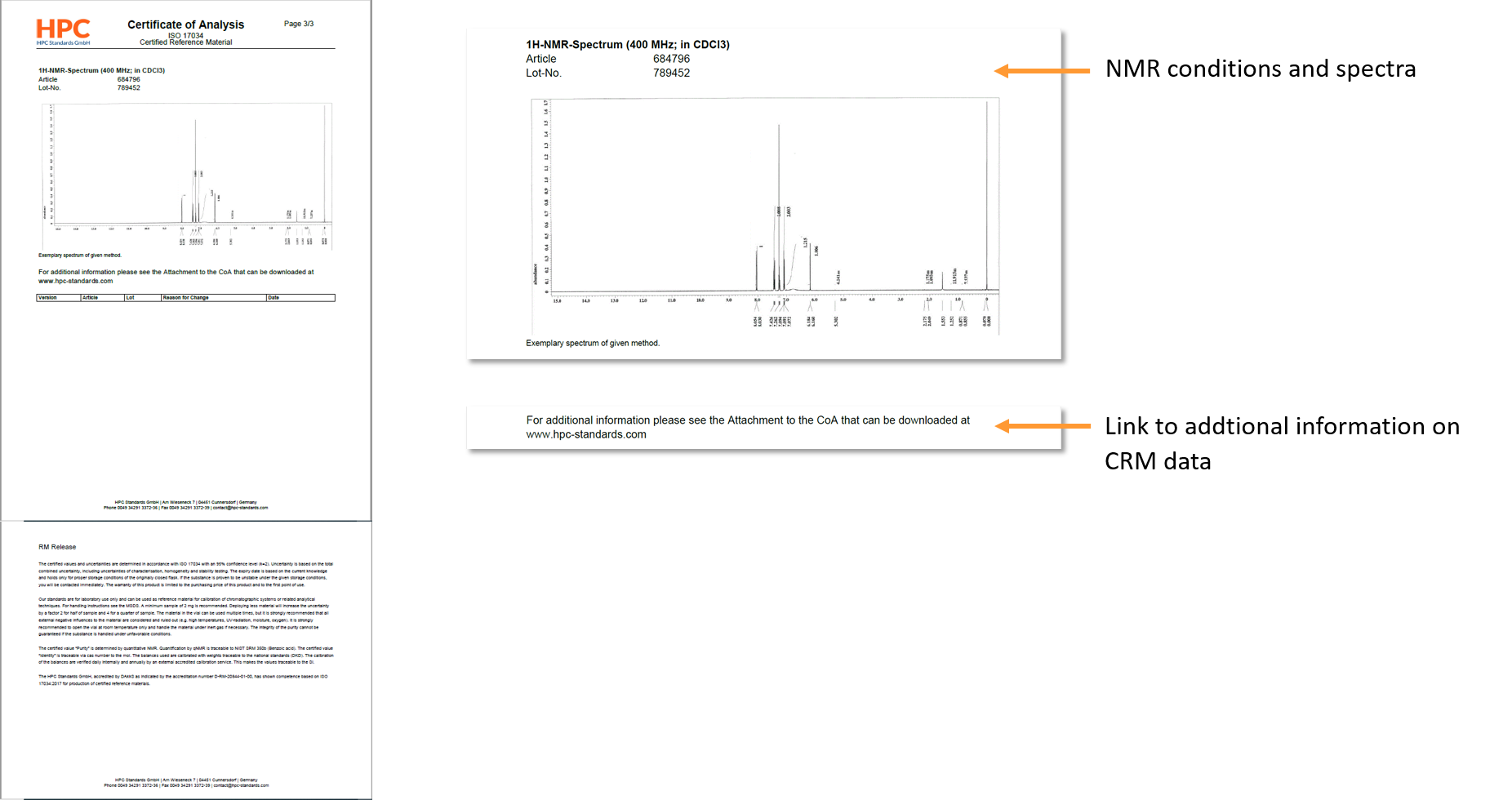
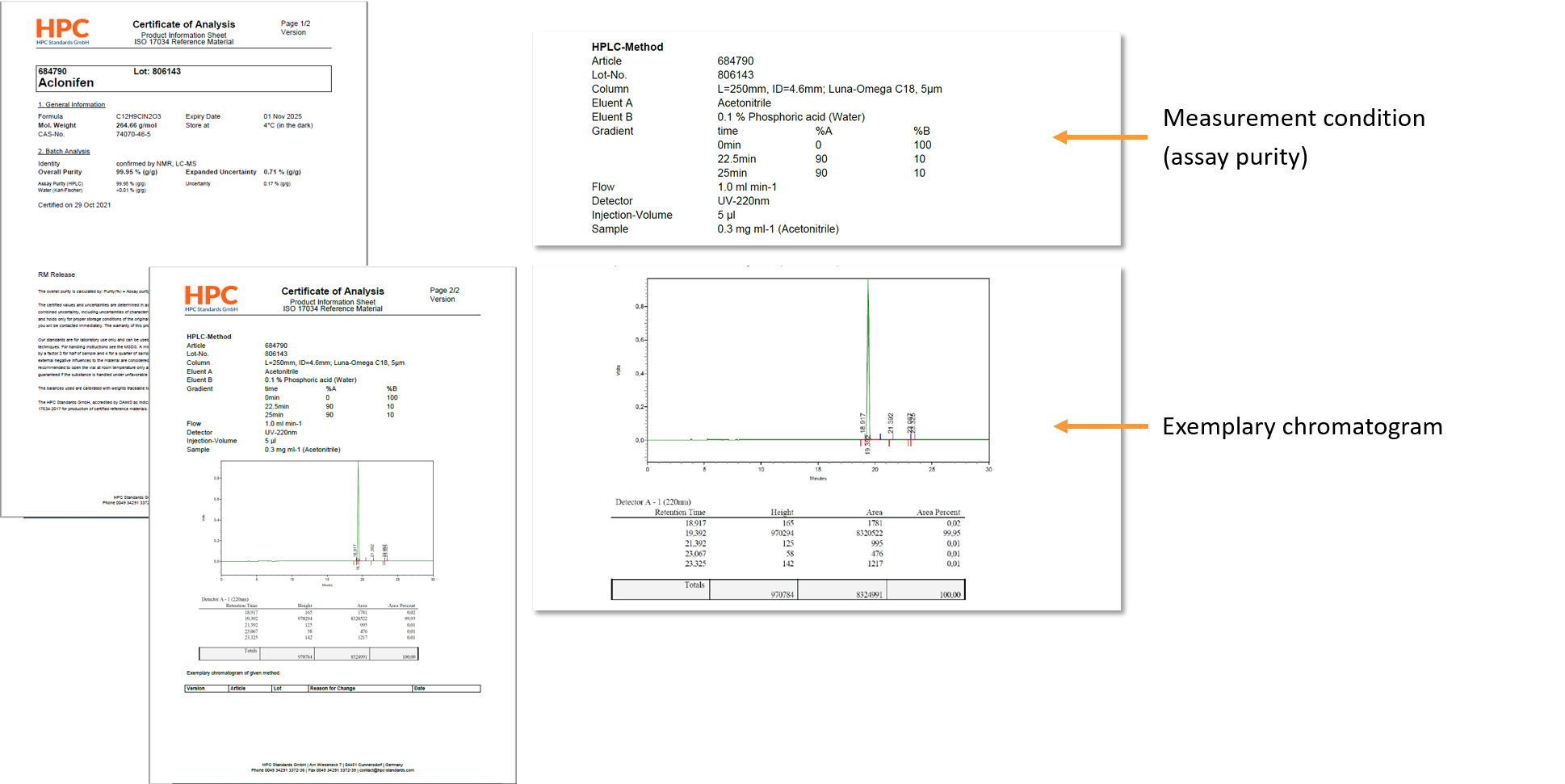
Additional information on the generation of data for our CRMs is available on our homepage (https://www.hpc-standards.com/coaattachment/). Here the applied procedures for traceability to NIST, calculation of uncertainty, homogeneity, characterization and stability (short term and long term) are described.