New user? / Forgot your password?
0 Item | 0,00 €
Bezafibrate Reference Materials
High-Purity Bezafibrate Reference Materials for Accurate Residue Analysis
Ensure precise and reliable residue analysis with HPC Standards GmbHs high-purity Bezafibrate reference materials. Our products meet international quality requirements and the highest industrial standards, making them essential for laboratories conducting food and environmental analysis. Trust HPC Standards GmbH for your analytical needs and regulatory compliance.
Product | Catalog No./ CAS No. | Quantity | Price | |
|---|---|---|---|---|
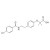 | 676317 | 1X100MG | Please log in. | |
Bezafibrate solution | 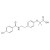 | 680846 | 1X1ML | Please log in. |
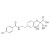 | 684245 | 1X10MG | Please log in. | |
D6-Bezafibrate solution | 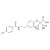 | 691034 | 1X1ML | Please log in. |
High-quality reference materials for accurate residue analysis of Bezafibrate.
Overview
Bezafibrate is a fibrate drug used primarily as a lipid-lowering agent to treat hyperlipidaemia. It is effective in reducing LDL cholesterol and triglycerides while increasing HDL levels in the blood. Bezafibrate was patented in 1971 and approved for medical use in 1978.
Uses
Bezafibrate is used to improve markers of combined hyperlipidemia, effectively reducing LDL and triglycerides and improving HDL levels. It is particularly beneficial for patients with metabolic syndrome and those with impaired glucose tolerance, as it may delay the progression to diabetes.
Regulatory
Bezafibrate is a prescription-only medication in the UK and US. It is identified by various codes such as CAS Number 41859-67-0, PubChem CID 39042, and DrugBank DB01393.
Monitoring
Regular monitoring of liver enzymes is essential due to the hepatic toxicity associated with Bezafibrate. Myopathy and, on rare occasions, rhabdomyolysis have also been reported.
Health Impact
Bezafibrate significantly reduces haemoglobin A1c (HbA1c) concentration in dyslipidemic patients with diabetes or hyperglycemia. It also shows potential in reducing tau protein hyperphosphorylation and other signs of tauopathy in transgenic mice.
Human Toxicity
The primary toxicity of Bezafibrate is hepatic, with abnormal liver enzymes being the most common issue. Myopathy and rhabdomyolysis are less common but serious side effects.
Environmental Impact
Studies on the environmental impact of Bezafibrate are limited. However, as with all pharmaceuticals, proper disposal and management are crucial to minimize any potential environmental effects.
Effects on Wildlife
There is limited data on the effects of Bezafibrate on wildlife. Further studies are needed to understand its impact fully.
Safety Measures
Proper handling and storage of Bezafibrate reference materials are essential to ensure safety and maintain the integrity of the product. Personal protective equipment (PPE) should be used when handling these materials.
Regulation
Bezafibrate is regulated as a prescription-only medication in many countries, including the UK and US. Compliance with local regulatory requirements is essential for laboratories using Bezafibrate reference materials.
Analytical Standards
HPC Standards GmbH provides high-purity reference materials for Bezafibrate, tested according to international quality requirements and meeting the highest industrial standards. These reference materials are essential for laboratories conducting food and environmental analysis to ensure compliance with regulatory limits.


