New user? / Forgot your password?
0 Item | 0,00 €
Metformin Hydrochloride
Ensure Accurate Residue Analysis with High-Purity Metformin Hydrochloride Reference Materials
HPC Standards GmbH offers top-tier, high-purity reference materials for Metformin Hydrochloride, essential for precise and reliable residue analysis. Our products meet international quality requirements and the highest industrial standards, making them indispensable for laboratories focused on food and environmental analysis to ensure regulatory compliance. Partner with us for unparalleled accuracy and reliability in your analytical processes.
Product | Catalog No./ CAS No. | Quantity | Price | |
|---|---|---|---|---|
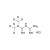 | 684244 | 1X5MG | Please log in. | |
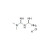 | 676327 | 1X250MG | Please log in. | |
Metformin hydrochloride solution | 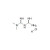 | 677784 | 1X1ML | Please log in. |
High-purity reference materials for accurate and reliable residue analysis of Metformin Hydrochloride.
Overview
Metformin Hydrochloride is a biguanide anti-hyperglycemic agent primarily used as a first-line medication for the treatment of type 2 diabetes. It is also used in the treatment of polycystic ovary syndrome (PCOS) and as an off-label adjunct to lessen the risk of metabolic syndrome in individuals taking antipsychotics.
Uses
Metformin Hydrochloride is used to lower blood glucose levels in individuals with type 2 diabetes and is also employed as a second-line agent for infertility in those with PCOS. It is sometimes used to reduce the risk of metabolic syndrome in patients on antipsychotic medications.
Regulatory
Metformin Hydrochloride is approved for use in many countries, including the US, UK, and Canada. It is available by prescription only and is listed on the World Health Organization's List of Essential Medicines.
Monitoring
Regular monitoring of blood glucose levels, kidney function, and vitamin B12 levels is recommended for individuals taking Metformin Hydrochloride to ensure efficacy and avoid potential adverse effects.
Health Impact
Human Toxicity
Metformin Hydrochloride is generally well-tolerated, but common adverse effects include gastrointestinal irritation such as diarrhea, nausea, and abdominal pain. Rare but serious adverse effects include lactic acidosis, particularly in individuals with severe kidney problems.
Environmental Impact
Effects on Wildlife
Metformin and its major transformation product, guanylurea, are present in wastewater treatment plant effluents and are regularly detected in surface waters. High concentrations have been reported in some rivers, potentially impacting aquatic life.
Safety Measures
To minimize adverse effects, it is recommended to start Metformin Hydrochloride at a low dose and gradually increase it. Individuals with severe renal impairment, known hypersensitivity to Metformin, or metabolic acidosis should avoid using this medication.
Regulation
Metformin Hydrochloride is regulated as a prescription-only medication in many countries. It is subject to strict quality control measures to ensure safety and efficacy.
Analytical Standards
HPC Standards GmbH provides high-purity reference materials for Metformin Hydrochloride, ensuring accurate and reliable residue analysis. Our products are tested according to international quality requirements and meet the highest industrial standards, making them essential for laboratories conducting food and environmental analysis to ensure compliance with regulatory limits.


