New user? / Forgot your password?
0 Item | 0,00 €
Sulfamethoxazole Reference Materials
Ensure Compliance with High-Purity Sulfamethoxazole Reference Materials
Unlock the accuracy and reliability your laboratory needs with our high-purity Sulfamethoxazole reference materials. Essential for food and environmental analysis, our products meet the highest industrial standards and international quality requirements. Trust HPC Standards GmbH to help you ensure compliance with regulatory limits and achieve precise residue analysis.
Product | Catalog No./ CAS No. | Quantity | Price | |
|---|---|---|---|---|
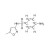 | 679495 | 1X10MG | Please log in. | |
13C6-Sulfamethoxazole solution | 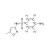 | 690840 | 1X1ML | Please log in. |
13C6-Sulfamethoxazole solution | 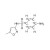 | 691569 | 1X1ML | Please log in. |
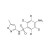 | 674800 | 1X10MG | Please log in. | |
D4-Sulfamethoxazole solution | 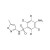 | 689549 | 1X1ML | Please log in. |
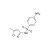 | 674029 | 1X250MG | Please log in. | |
Sulfamethoxazole solution | 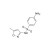 | 679816 | 1X1ML | Please log in. |
ISO 17034 Certified Reference Material Sulfamethoxazole solution | 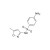 | 686237 | 1X1ML | Please log in. |
High-purity reference materials for accurate and reliable residue analysis of Sulfamethoxazole.
Overview
Sulfamethoxazole is a sulfonamide antibiotic used to treat bacterial infections such as urinary tract infections, bronchitis, and prostatitis. It is effective against both gram-negative and gram-positive bacteria, including Escherichia coli and Listeria monocytogenes.
Our high-purity reference materials for Sulfamethoxazole are essential for laboratories conducting food and environmental analysis to ensure compliance with regulatory limits.
Uses
Sulfamethoxazole is primarily used in combination with trimethoprim (SMX-TMP) for the treatment of various bacterial infections. This combination is listed on the WHO Model List of Essential Medicines as a first-choice treatment for urinary tract infections.
It is administered orally or intravenously and is well-absorbed in the body, distributing into most body tissues, including sputum, vaginal fluid, and middle ear fluid.
Regulatory
Sulfamethoxazole is a prescription-only medication in many countries, including Australia (S4), Canada (Rx-only), the UK (POM), and the US (Rx-only). It is important for laboratories to use high-purity reference materials to ensure compliance with these regulatory standards.
Monitoring
Monitoring the residue levels of Sulfamethoxazole in food and environmental samples is crucial for public health and safety. Our reference materials provide accurate and reliable results, helping laboratories meet international quality requirements.
Health Impact
Common side effects of Sulfamethoxazole include nausea, vomiting, loss of appetite, and skin rashes. Severe adverse reactions, although rare, can include Stevens-Johnson syndrome, toxic epidermal necrolysis, and various blood dyscrasias.
It is contraindicated in individuals with known hypersensitivity to trimethoprim or sulfonamides.
Environmental Impact
Sulfamethoxazole can enter the environment through various pathways, including pharmaceutical manufacturing and improper disposal. Monitoring its levels in environmental samples is essential to prevent potential ecological harm.
Safety Measure
Proper handling and disposal of Sulfamethoxazole are necessary to minimize exposure and environmental contamination. Laboratories should follow safety guidelines and use high-purity reference materials to ensure accurate analysis.
Analytical Standards
Our high-purity reference materials for Sulfamethoxazole are tested according to international quality requirements and meet the highest industrial standards. These materials are essential for laboratories conducting food and environmental analysis to ensure compliance with regulatory limits.
We provide reference materials for pesticides, veterinary products, their metabolites, and stable isotope-labelled derivatives, making us your competent partner in the fields of food and environmental analysis.


