New user? / Forgot your password?
0 Item | 0,00 €
Pazufloxacin Mesylate
Ensure Accurate Residue Analysis with High-Purity Pazufloxacin Mesylate Reference Materials
Pazufloxacin mesylate is a crucial fluoroquinolone antibiotic for treating bacterial infections and is essential for residue analysis in food and environmental samples. HPC Standards GmbH offers high-purity reference materials for pazufloxacin mesylate, tested to meet international quality standards. Our products support laboratories in achieving precise quantification and compliance with regulatory limits, ensuring public health and safety. Trust HPC Standards GmbH as your competent partner in food and environmental analysis.
Product | Catalog No./ CAS No. | Quantity | Price | |
|---|---|---|---|---|
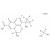 | 691895 | 1X5MG | ||
D4-Pazufloxacin mesylate solution | 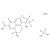 | 691912 | 1X1ML | |
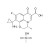 | 689008 | 1X100MG | Please log in. |
High-Purity Reference Materials for Accurate Residue Analysis
Overview
Pazufloxacin mesylate is a fluoroquinolone antibiotic used in the treatment of various bacterial infections. It is known for its broad-spectrum activity against Gram-positive and Gram-negative bacteria. Pazufloxacin mesylate is commonly used in clinical settings and is essential for laboratories conducting residue analysis in food and environmental samples.
Uses
Pazufloxacin mesylate is primarily used in the medical field to treat bacterial infections. It is also utilized in veterinary medicine for the treatment of infections in animals. In analytical laboratories, it serves as a reference material for the accurate quantification of residues in food and environmental samples.
Regulatory
The use of pazufloxacin mesylate is regulated by various international bodies to ensure its safe application in both human and veterinary medicine. Compliance with regulatory limits is crucial for laboratories conducting residue analysis to ensure public health and safety.
Monitoring
Regular monitoring of pazufloxacin mesylate residues in food and environmental samples is essential to ensure compliance with regulatory standards. Analytical methods such as high-performance liquid chromatography (HPLC) are commonly used for this purpose.
Health Impact
Human Toxicity
Pazufloxacin mesylate, like other fluoroquinolones, can cause side effects in humans, including gastrointestinal disturbances, central nervous system effects, and hypersensitivity reactions. It is important to adhere to prescribed dosages to minimize potential health risks.
Environmental Impact
Effects on Wildlife
Residues of pazufloxacin mesylate can have adverse effects on wildlife, particularly aquatic organisms. Monitoring and controlling the levels of this antibiotic in the environment are crucial to prevent ecological harm.
Safety Measures
Proper handling and storage of pazufloxacin mesylate are essential to ensure safety in laboratory settings. Personal protective equipment (PPE) and adherence to safety protocols are recommended to minimize exposure risks.
Regulation
Regulatory agencies such as the FDA, EMA, and others have set maximum residue limits (MRLs) for pazufloxacin mesylate in food products. Laboratories must ensure that their analytical methods are compliant with these regulations to guarantee accurate and reliable results.
Analytical Standards
HPC Standards GmbH provides high-purity reference materials for pazufloxacin mesylate, tested according to international quality requirements. These reference materials are essential for laboratories to achieve accurate and reliable residue analysis, ensuring compliance with regulatory limits.


