New user? / Forgot your password?
0 Item | 0,00 €
Captan
Ensure Accurate Residue Analysis with High-Purity Captan Reference Materials
Captan is a widely used fungicide essential for controlling fungal diseases in agriculture. HPC Standards GmbH offers high-purity reference materials for Captan, ensuring precise and reliable residue analysis. Our products meet international quality standards, making them indispensable for laboratories focused on food and environmental analysis. Trust HPC Standards GmbH for compliance with regulatory limits and superior analytical performance.
Product | Catalog No./ CAS No. | Quantity | Price | |
|---|---|---|---|---|
ISO 17034 Reference Material | 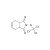 | 672819 | 1X250MG | Please log in. |
Captan solution | 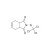 | 673500 | 1X5ML | Please log in. |
Captan solution | 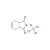 | 678268 | 1X5ML | Please log in. |
ISO 17034 Certified Reference Material | 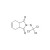 | 691289 | 1X100MG | Please log in. |
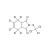 | 674631 | 1X10MG | Please log in. | |
D6-Captan solution | 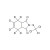 | 674637 | 1X1ML | Please log in. |
D6-Captan solution | 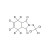 | 692423 | 1X1ML | Please log in. |
High-purity reference materials for accurate residue analysis
Overview
Captan is a general use pesticide (GUP) that belongs to the phthalimide class of fungicides. It is a white solid, although commercial samples often appear yellow or brownish. Captan is widely used in agriculture to control fungal diseases on fruits, vegetables, and ornamental plants.
Uses
Captan is primarily used to control diseases on a variety of fruits and vegetables, as well as ornamental plants. It is often applied during apple production and is effective against certain oomycetes, such as Pythium, making it useful for controlling damping off. Captan is utilized by both home and agricultural growers.
Regulatory
Captan was previously classified as a probable human carcinogen by the US Environmental Protection Agency (EPA). However, it was reclassified in 2004. The EPA now considers Captan to be a potential carcinogen only at prolonged high doses that cause cytotoxicity and regenerative cell hyperplasia. When used according to approved product labels, Captan is not likely to pose cancer risks of concern.
Monitoring
Monitoring of Captan residues is essential for ensuring compliance with regulatory limits. Laboratories conducting food and environmental analysis rely on high-purity reference materials to achieve accurate and reliable results.
Health Impact
Captan has been deemed not likely to cause tumors at doses that do not irritate the intestine. High doses of Captan, which are many orders of magnitude above those likely to be consumed in the diet or encountered occupationally, are required to pose a carcinogenic risk. Captan is not mutagenic in vivo.
Environmental Impact
Captan biodegrades with a half-life of less than one day in soil, reducing its environmental persistence. However, its use must be carefully managed to prevent potential negative impacts on non-target organisms and ecosystems.
Safety Measure
Captan is combustible and considered a potential occupational carcinogen. Safety measures include adhering to recommended exposure limits and using personal protective equipment (PPE) to minimize exposure. The NIOSH recommends a TWA (time-weighted average) of 5 mg/m³.
Analytical Standards
HPC Standards GmbH provides high-purity reference materials for Captan, ensuring accurate and reliable residue analysis. Our products are tested according to international quality requirements and meet the highest industrial standards. These reference materials are essential for laboratories conducting food and environmental analysis to ensure compliance with regulatory limits.


