New user? / Forgot your password?
0 Item | 0,00 €
Diclofenac Sodium Reference Materials
Ensure Accurate Residue Analysis with High-Purity Diclofenac Sodium Reference Materials
At HPC Standards GmbH, we offer premium Diclofenac Sodium reference materials that guarantee precise and reliable residue analysis. Our products are meticulously tested to meet international quality standards, making them indispensable for laboratories focused on food and environmental analysis. Trust HPC Standards for your high-purity analytical needs and ensure compliance with regulatory limits.
Product | Catalog No./ CAS No. | Quantity | Price | |
|---|---|---|---|---|
13C6-Diclofenac sodium solution | 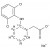 | 687037 | 1X1ML | Please log in. |
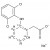 | 687344 | 1X10MG | Please log in. | |
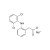 | 673070 | 1X100MG | Please log in. | |
Diclofenac sodium solution | 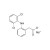 | 680861 | 1X1ML | Please log in. |
High-purity reference materials for accurate and reliable residue analysis.
Overview
Diclofenac sodium is a nonsteroidal anti-inflammatory drug (NSAID) used to treat pain and inflammatory diseases such as arthritis, dysmenorrhea, and gout. It is available in various forms including oral, rectal, intramuscular, intravenous, and topical applications.
Uses
Diclofenac sodium is commonly used to manage pain related to arthritis, dysmenorrhea, and other inflammatory disorders. It is also used for treating mild to moderate postoperative or post-traumatic pain, actinic keratosis, and acute pain caused by minor strains, sprains, and contusions.
In veterinary medicine, it is used to treat bacterial diseases in livestock, although its use is banned in some countries due to its environmental impact.
Regulatory
Diclofenac sodium is available by prescription in most countries. In the UK, oral preparations are classified as prescription-only medicines, while topical preparations are available over-the-counter.
In some countries, its use in veterinary medicine is banned due to its harmful effects on wildlife, particularly vultures.
Monitoring
Monitoring of diclofenac sodium includes regular liver function tests during long-term treatment due to potential hepatotoxicity. Patients receiving long-term therapy should have their transaminases measured periodically.
Health Impact
Human Toxicity
Common side effects include gastrointestinal issues such as abdominal pain and bleeding, as well as cardiovascular risks including heart disease and stroke. It is not recommended for use during the third trimester of pregnancy.
Environmental Impact
Effects on Wildlife
Diclofenac has been shown to cause significant harm to wildlife, particularly vultures. The drug has led to a sharp decline in vulture populations in the Indian subcontinent due to kidney failure from consuming treated livestock carcasses.
It also affects freshwater fish species, causing damage to internal organs such as gills, kidneys, and liver.
Safety Measures
Diclofenac sodium should be used with caution in patients with pre-existing liver or kidney conditions, and its use should be avoided in patients with severe heart conditions. Gastro-protective drugs are often prescribed during long-term treatment to mitigate gastrointestinal side effects.
Regulation
Due to its environmental impact, diclofenac sodium is banned for veterinary use in several countries. In the European Union, it is on the watch list for pollutants affecting the Baltic Sea.
Analytical Standards
HPC Standards GmbH provides high-purity reference materials for diclofenac sodium, ensuring accurate and reliable residue analysis. Our products meet international quality requirements and the highest industrial standards, making them essential for laboratories conducting food and environmental analysis to ensure compliance with regulatory limits.


