New user? / Forgot your password?
0 Item | 0,00 €
Ethynylestradiol Reference Materials
Unlock Precision in Residue Analysis with High-Purity Ethynylestradiol Reference Materials
Ensure your laboratorys accuracy and compliance with HPC Standards GmbHs high-purity Ethynylestradiol reference materials. Our products meet the highest international quality standards, making them indispensable for food and environmental analysis. Trust in our expertise to deliver reliable results for your residue analysis needs.
Product | Catalog No./ CAS No. | Quantity | Price | |
|---|---|---|---|---|
13C2-17-alpha-Ethinylestradiol solution | 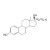 | 683803 | 1X1ML | Please log in. |
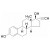 | 672998 | 1X250MG | Please log in. | |
17a-Ethinylestradiol solution | 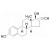 | 677782 | 1X1ML | Please log in. |
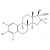 | 687159 | 1X5MG | Please log in. | |
D4-17-alpha-Ethinylestradiol solution |  | 687160 | 1X1ML | Please log in. |
High-Purity Reference Materials for Accurate Residue Analysis
Overview
Ethynylestradiol (EE) is a synthetic estrogen widely used in combination with progestins in birth control pills. It is also used in menopausal hormone therapy and the treatment of certain hormone-sensitive cancers. EE is known for its high oral bioavailability and resistance to metabolism, making it a prevalent choice in contraceptive formulations.
Uses
EE is primarily used in combined oral contraceptives (COCs) to prevent pregnancy. It is also utilized in menopausal hormone therapy to alleviate symptoms such as hot flashes and vaginal dryness. Additionally, EE has applications in treating hypogonadism, osteoporosis prevention, and as part of feminizing hormone therapy for transgender women.
Regulatory
EE is a prescription-only medication with stringent regulatory controls due to its potent biological effects and potential side effects. It is included in various international pharmacopeias and must meet rigorous quality standards.
Monitoring
Regular monitoring of EE levels is essential in clinical settings to ensure therapeutic efficacy and minimize adverse effects. Analytical methods such as liquid chromatography-mass spectrometry (LC-MS) are commonly used for this purpose.
Health Impact
Human Toxicity
Common side effects of EE include breast tenderness, headache, fluid retention, and nausea. In men, it can cause feminization and hypogonadism. Serious but rare side effects include blood clots, liver damage, and uterine cancer.
Environmental Impact
Effects on Wildlife
EE can enter freshwater ecosystems through wastewater, affecting fish and amphibian populations. Chronic exposure can lead to reproductive issues and population declines in aquatic species.
Safety Measures
Proper handling and disposal of EE are crucial to minimize environmental contamination. Laboratories must follow safety protocols to protect personnel and the environment.
Regulation
EE is regulated under various national and international guidelines, including those from the FDA and EMA. Compliance with these regulations ensures the safe and effective use of EE in medical and research applications.
Analytical Standards
HPC Standards GmbH provides high-purity reference materials for EE, tested according to international quality requirements. These materials are essential for accurate residue analysis in food and environmental samples, ensuring compliance with regulatory limits.


