New user? / Forgot your password?
0 Item | 0,00 €
Cephacetrile Reference Materials
Ensure Accurate Residue Analysis with High-Purity Cephacetrile Reference Materials
Cephacetrile, a broad-spectrum first-generation cephalosporin antibiotic, is essential for treating bacterial infections in veterinary medicine. HPC Standards GmbH offers high-purity reference materials for Cephacetrile, ensuring precise and reliable residue analysis. Our products meet the highest industrial standards, making them indispensable for laboratories focused on food and environmental analysis to comply with regulatory limits. Trust HPC Standards GmbH for your Cephacetrile reference material needs.
Product | Catalog No./ CAS No. | Quantity | Price | |
|---|---|---|---|---|
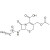 | 679544 | 1X10MG | Please log in. | |
13C3-Cephacetrile solution | 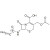 | 690888 | 1X1ML | |
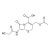 | 679543 | 1X10MG | Please log in. | |
Cephacetrile solution | 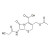 | 690887 | 1X1ML | Please log in. |
High-purity reference materials for accurate and reliable residue analysis of Cephacetrile.
Overview
Cephacetrile, also known as Cefacetrile, is a broad-spectrum first-generation cephalosporin antibiotic. It is effective against both gram-positive and gram-negative bacterial infections. Cephacetrile is commonly used under trade names such as Celospor, Celtol, and Cristacef, and is particularly utilized in veterinary medicine for treating mammary infections in lactating cows.
Uses
Cephacetrile is primarily used in the treatment of bacterial infections. It is administered intravenously, intramuscularly, or intramammarily. In veterinary medicine, it is used to treat mammary infections in dairy cows, ensuring the health and productivity of livestock.
Regulatory
Cephacetrile is categorized under the ATC codes J01DB10 and QJ51DB10 by the WHO. Its legal status is prescription-only (℞), meaning it must be prescribed by a licensed healthcare professional.
Monitoring
Regular monitoring of Cephacetrile residues in food and the environment is crucial to ensure compliance with regulatory limits. High-purity reference materials are essential for laboratories to conduct accurate and reliable residue analysis.
Health Impact
Human Toxicity
Cephacetrile is a bacteriostatic antibiotic, meaning it inhibits the growth and reproduction of bacteria. Its protein binding ranges from 23% to 38%, and it has a half-life of approximately 1.2 hours. The primary route of excretion is renal, with about 72% being excreted through the kidneys.
Environmental Impact
Effects on Wildlife
The use of Cephacetrile in veterinary medicine can lead to its presence in the environment, potentially affecting wildlife. Proper disposal and management practices are necessary to minimize environmental contamination.
Safety Measures
Handling Cephacetrile requires adherence to safety guidelines to prevent accidental exposure and contamination. Personal protective equipment (PPE) should be used, and proper disposal procedures should be followed.
Regulation
Cephacetrile is regulated to ensure its safe and effective use. Laboratories must comply with international quality requirements when using reference materials for residue analysis.
Analytical Standards
HPC Standards GmbH provides high-purity reference materials for Cephacetrile, ensuring accurate and reliable residue analysis. Our products meet the highest industrial standards and are essential for laboratories conducting food and environmental analysis to ensure compliance with regulatory limits.


