New user? / Forgot your password?
0 Item | 0,00 €
Nitrofurazone Reference Materials
Ensure Accurate Residue Analysis with High-Purity Nitrofurazone Reference Materials
HPC Standards GmbH offers top-tier Nitrofurazone reference materials, essential for precise residue analysis in food and environmental laboratories. Our products meet international quality standards, providing reliable results to ensure regulatory compliance. Trust HPC Standards for your analytical needs in detecting Nitrofurazone residues.
Product | Catalog No./ CAS No. | Quantity | Price | |
|---|---|---|---|---|
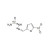 | 679166 | 1X10MG | Please log in. | |
13C,15N2-Nitrofurazone solution | 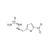 | 690811 | 1X1ML | Please log in. |
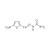 | 672875 | 1X250MG | Please log in. | |
Nitrofurazone solution | 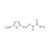 | 686130 | 1X5ML |
High-purity reference materials for accurate and reliable residue analysis of Nitrofurazone.
Overview
Nitrofurazone is an antimicrobial organic compound belonging to the nitrofuran class. It is most commonly used as a topical antibiotic ointment effective against gram-positive and gram-negative bacteria, and can be used in the treatment of trypanosomiasis.
Uses
Nitrofurazone was previously used in the U.S. as a prescription topical solution, cream, or ointment for treating bacterial skin infections, wounds, burns, and ulcers. It was also used prophylactically to prevent infection in skin grafts. Currently, it is used in veterinary medicine for treating superficial bacterial infections, burns, and cutaneous ulcers in dogs, cats, and horses. It is also used to treat infections in ornamental fish.
Regulatory
Nitrofurazone use in medicine has decreased due to the availability of safer and more effective products. It is listed under California Proposition 65 as a substance with clear evidence of mutagenic and carcinogenic properties from animal studies. Its use has been discontinued for human use in the USA. The use of nitrofurazone in animals raised for human consumption is strictly banned.
Monitoring
Monitoring of nitrofurazone residues is crucial for ensuring compliance with regulatory limits, especially in food and environmental analysis. Laboratories require high-purity reference materials to perform accurate residue analysis.
Health Impact
Human Toxicity
Nitrofurazone is suspected to be a human carcinogen. It has been shown to induce mammary tumors in rats and ovarian tumors in mice. Adverse effects from topical use include erythema, pruritus, dermatitis, rash, edema, or inflammation. It is contraindicated for people with chronic kidney disease and large total body surface area burns due to the presence of polyethylene glycol in topical preparations.
Environmental Impact
Effects on Wildlife
Nitrofurazone has been found in honey, meat, and seafood, raising concerns about its impact on wildlife and the environment. Its use in animals for human consumption is banned to prevent contamination.
Safety Measures
Proper personal protective equipment should be utilized when handling nitrofurazone. Due to its potential carcinogenic and mutagenic properties, strict safety protocols must be followed.
Analytical Standards
HPC Standards GmbH provides high-purity reference materials for Nitrofurazone, ensuring accurate and reliable residue analysis. Our products meet the highest industrial standards and are essential for laboratories conducting food and environmental analysis to ensure compliance with regulatory limits.


