New user? / Forgot your password?
0 Item | 0,00 €
Methanol Reference Materials
High-Purity Methanol Reference Materials for Accurate Residue Analysis
Ensure precision and reliability in your residue analysis with our high-purity Methanol reference materials. Essential for laboratories focused on food and environmental analysis, our Methanol standards meet the highest international quality requirements and industrial standards. Trust HPC Standards GmbH for your analytical needs and ensure compliance with regulatory limits.
Product | Catalog No./ CAS No. | Quantity | Price | |
|---|---|---|---|---|
D4-Methanol solution | 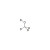 | 677808 | 1X1ML | Please log in. |
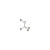 | 688340 | 1X100MG | Please log in. | |
 | 678017 | 1X5ML | Please log in. |
High-purity reference materials for accurate residue analysis
Overview
Methanol, also known as methyl alcohol or wood spirit, is the simplest aliphatic alcohol with the chemical formula CH3OH. It is a light, volatile, colorless, and flammable liquid with a distinctive alcoholic odor similar to ethanol but is more acutely toxic.
Methanol is primarily produced industrially by hydrogenation of carbon monoxide. It serves as a precursor to various commodity chemicals, including formaldehyde, acetic acid, and methyl tert-butyl ether.
Uses
Methanol is widely used in the production of formaldehyde, acetic acid, and methyl tert-butyl ether (MTBE), which is a major octane booster in gasoline. It is also used in the synthesis of other chemicals, including methylamines, methyl halides, and methyl ethers.
Additionally, methanol is used as a fuel, solvent, antifreeze, and denaturant for ethanol. It has potential applications as an energy carrier and in direct-methanol fuel cells for consumer electronics.
Regulatory
Methanol is regulated due to its toxicity and flammability. It is classified under various regulatory frameworks, including GHS labelling with hazard statements such as H225, H301, H311, H331, and H370.
Occupational safety guidelines are provided by organizations like NIOSH, which sets permissible exposure limits (PEL) and recommended exposure limits (REL) for methanol.
Monitoring
Monitoring methanol levels in industrial and laboratory settings is crucial for safety. Methods include gas chromatography and UV-vis spectroscopy to detect impurities and ensure compliance with safety standards.
Health Impact
Human Toxicity
Methanol is highly toxic to humans. Ingestion of as little as 10 mL can cause permanent blindness, and 30 mL can be fatal. It affects the central nervous system and is metabolized to formaldehyde and formic acid, which are toxic to the optic nerve and other organs.
Environmental Impact
Effects on Wildlife
Methanol is biodegradable and has a low environmental toxicity. It does not persist in aerobic or anaerobic environments and is unlikely to accumulate in groundwater, surface water, air, or soil.
Safety Measures
Methanol is highly flammable and should be handled with care. Safety measures include using appropriate personal protective equipment (PPE), proper ventilation, and storage in flame-proof containers. Methanol fires should be extinguished with dry chemical, carbon dioxide, water spray, or alcohol-resistant foam.
Analytical Standards
Our high-purity methanol reference materials meet international quality requirements and the highest industrial standards. These materials are essential for laboratories conducting food and environmental analysis to ensure compliance with regulatory limits.
Typical impurities in methanol include water, acetone, and ethanol. Water content can be determined by Karl-Fischer titration, and aromatic impurities can be detected using UV-vis spectroscopy.


