New user? / Forgot your password?
0 Item | 0,00 €
Febantel Reference Materials
Ensure Accurate Residue Analysis with High-Purity Febantel Reference Materials
HPC Standards GmbH offers high-purity reference materials for Febantel, essential for precise residue analysis in food and environmental laboratories. Our products meet international quality standards, ensuring compliance with regulatory limits and safeguarding consumer health. Trust HPC Standards for reliable and accurate analytical solutions in veterinary medicine.
Product | Catalog No./ CAS No. | Quantity | Price | |
|---|---|---|---|---|
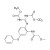 | 679402 | 1X10MG | Please log in. | |
D6-Febantel solution | 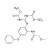 | 693362 | 1X1ML | Please log in. |
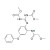 | 676824 | 1X100MG | Please log in. | |
Febantel solution | 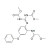 | 682381 | 1X5ML | Please log in. |
High-purity reference materials for accurate residue analysis of Febantel.
Overview
Febantel is a broad-spectrum anthelmintic used in veterinary medicine to treat parasitic worm infections in animals. It is commonly used in combination with other anthelmintics to enhance efficacy.
Uses
Febantel is primarily used in the veterinary field for the treatment of gastrointestinal nematodes and cestodes in animals such as dogs, cats, and livestock. It is often included in combination products to provide a wider spectrum of activity against various parasites.
Regulatory
Febantel is regulated by various international and national agencies to ensure its safe and effective use in veterinary medicine. Compliance with these regulations is essential for the approval and distribution of veterinary products containing Febantel.
Monitoring
Regular monitoring of Febantel residues in food-producing animals is crucial to ensure that residue levels remain within the established safety limits. This monitoring helps protect consumer health and maintain confidence in the safety of animal-derived food products.
Health Impact
Human toxicity
Febantel is generally considered safe for use in animals when administered according to prescribed dosages. However, improper use or overexposure may pose health risks. It is important for veterinary professionals to follow recommended guidelines to minimize any potential human health risks.
Environmental Impact
Effects on wildlife
As with many veterinary pharmaceuticals, the environmental impact of Febantel should be considered. Residues from treated animals can enter the environment and potentially affect wildlife. Proper waste management and disposal practices are necessary to mitigate these risks.
Safety Measure
When handling Febantel, it is important to follow appropriate safety measures, including the use of personal protective equipment (PPE) and adherence to handling guidelines. This ensures the safety of both the handler and the environment.
Regulation
Febantel is subject to stringent regulatory controls to ensure its safety, efficacy, and quality. Regulatory agencies establish maximum residue limits (MRLs) and other guidelines to govern its use in veterinary medicine.
Analytical Standards
HPC Standards GmbH provides high-purity reference materials for Febantel, ensuring accurate and reliable residue analysis. Our products are tested according to international quality requirements and meet the highest industrial standards. These reference materials are essential for laboratories conducting food and environmental analysis to ensure compliance with regulatory limits.


