New user? / Forgot your password?
0 Item | 0,00 €
Stanozolol Reference Materials
High-Purity Stanozolol Reference Materials for Accurate Residue Analysis
Ensure the highest accuracy and reliability in your residue analysis with HPC Standards GmbHs high-purity Stanozolol reference materials. Our products are essential for laboratories conducting food and environmental analysis, meeting the highest industrial standards and international quality requirements. Trust HPC Standards GmbH to be your competent partner in achieving compliance with regulatory limits.
Product | Catalog No./ CAS No. | Quantity | Price | |
|---|---|---|---|---|
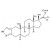 | 693173 | 1X5MG | ||
D3-Stanozolol solution | 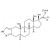 | 693174 | 1X1ML | |
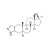 | 674902 | 1X100MG | Please log in. | |
Stanozolol solution | 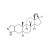 | 680435 | 1X1ML | Please log in. |
High-purity reference materials for accurate and reliable residue analysis in food and environmental samples.
Overview
Stanozolol, also known as Winstrol among other names, is a synthetic androgen and anabolic steroid (AAS) derived from dihydrotestosterone (DHT). Developed by Winthrop Laboratories in 1962, it has been used in both human and veterinary medicine. Stanozolol is notable for its high oral bioavailability and is commonly used in veterinary medicine as well as for performance enhancement in sports.
Uses
Stanozolol has been used to treat hereditary angioedema, venous insufficiency, osteoporosis, and skeletal muscle injury. It is also used for physique- and performance-enhancing purposes by competitive athletes, bodybuilders, and powerlifters. In veterinary medicine, it is used to stimulate the formation of red blood cells, arouse appetite, and promote weight gain in animals.
Regulatory
Stanozolol is a controlled substance in many countries. In the United States, it is classified as a Schedule III controlled substance under the Anabolic Steroids Control Act of 1990. It is also banned from use in sports competition under the World Anti-Doping Agency (WADA) regulations.
Monitoring
Methods for detecting stanozolol in body fluids typically involve gas chromatography-mass spectrometry or liquid chromatography-mass spectrometry. These methods can detect stanozolol metabolites in urine for up to 10 days after administration.
Health Impact
Human Toxicity
Side effects of stanozolol include virilization, hepatotoxicity, cardiovascular disease, and hypertension. Due to its 17α-methyl group, stanozolol is orally active but also hepatotoxic.
Environmental Impact
Effects on Wildlife
The use of anabolic steroids like stanozolol in veterinary medicine can lead to environmental contamination, potentially affecting wildlife. Proper disposal and monitoring are essential to minimize environmental risks.
Safety Measures
Proper handling and disposal of stanozolol are crucial to ensure safety. Laboratories should follow standard safety protocols and regulations to prevent exposure and environmental contamination.
Regulation
Stanozolol is regulated under various national and international laws. Compliance with these regulations is essential for legal use and distribution. Laboratories and companies must adhere to these regulations to ensure safe and lawful operations.
Analytical Standards
HPC Standards GmbH provides high-purity reference materials for stanozolol, ensuring accurate and reliable residue analysis. Our products meet the highest industrial standards and are essential for laboratories conducting food and environmental analysis to ensure compliance with regulatory limits.


