New user? / Forgot your password?
0 Item | 0,00 €
Trimethoprim Reference Materials
Ensure Accurate Residue Analysis with High-Purity Trimethoprim Reference Materials
HPC Standards GmbH offers top-tier Trimethoprim reference materials, meticulously tested to meet international quality standards. Essential for laboratories focused on food and environmental analysis, our products guarantee precise and reliable results, ensuring compliance with regulatory limits. Partner with us for unparalleled expertise in high-purity analytical standards.
Product | Catalog No./ CAS No. | Quantity | Price | |
|---|---|---|---|---|
 | 690407 | 1X10MG | Please log in. | |
13C3-Trimethoprim solution | 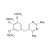 | 690443 | 1X1ML | Please log in. |
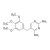 | 679828 | 1X10MG | Please log in. | |
D9-Trimethoprim solution | 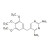 | 687614 | 1X1ML | Please log in. |
ISO 17034 Reference Material | 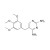 | 672980 | 1X250MG | Please log in. |
Trimethoprim solution | 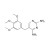 | 679824 | 1X1ML | Please log in. |
ISO 17034 Certified Reference Material | 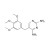 | 691054 | 1X100MG | Please log in. |
High-purity reference materials for accurate and reliable residue analysis of Trimethoprim.
Overview
Trimethoprim is an antibiotic primarily used to treat bladder infections, middle ear infections, and travelers' diarrhea. It is also used in combination with sulfamethoxazole or dapsone for the treatment of Pneumocystis pneumonia in individuals with HIV/AIDS. Trimethoprim works by inhibiting bacterial dihydrofolate reductase, leading to the prevention of bacterial DNA and RNA synthesis.
Uses
Trimethoprim is mainly used for the treatment of urinary tract infections and can be effective against various aerobic bacterial species. It is also employed in the prevention and treatment of Pneumocystis jirovecii pneumonia. However, it is generally not recommended for anaerobic infections such as Clostridium difficile colitis.
Regulatory
Trimethoprim is available only by prescription in many countries, including Australia (S4), Canada (℞-only), the UK (POM), and the US (℞-only). It is listed on the World Health Organization's List of Essential Medicines and is available as a generic medication.
Monitoring
It is crucial to perform cultures and susceptibility tests to ensure that the bacteria are susceptible to trimethoprim. This helps in effective treatment and prevents resistance development.
Health Impact
Human Toxicity
Common side effects include nausea, changes in taste, rash, vomiting, diarrhea, sun sensitivity, and itchiness. Rare side effects include blood problems such as thrombocytopenia and megaloblastic anemia, hyperkalemia, and artificial rise in serum creatinine levels. Trimethoprim may also increase the expression of Shiga toxin in EHEC infections.
Environmental Impact
Effects on Wildlife
As an antibiotic, trimethoprim can potentially affect aquatic and terrestrial ecosystems if not properly managed. Monitoring and regulation are essential to mitigate its environmental impact.
Safety Measures
Trimethoprim should be used with caution in individuals with known hypersensitivity to the drug or a history of megaloblastic anemia due to folate deficiency. It is also advised to avoid alcohol consumption during treatment, especially when used in combination with sulfamethoxazole.
Regulation
Trimethoprim is regulated under prescription-only status in various countries. It is essential to comply with local regulatory requirements for its use and distribution.
Analytical Standards
HPC Standards GmbH provides high-purity reference materials for Trimethoprim to ensure accurate and reliable residue analysis. Our products meet international quality requirements and the highest industrial standards, making them essential for laboratories conducting food and environmental analysis to ensure compliance with regulatory limits.


