New user? / Forgot your password?
0 Item | 0,00 €
Product groups
- A
- Abamectin
- Abscisic acid
- Acenaphthene
- Acenaphthylene
- Acephate
- Acequinocyl
- Acequinocyl-hydroxy
- Acesulfame K
- Acetaldehyde-2,4-DNPH
- Acetamidoantipyrine
- Acetaminophen
- Acetamiprid
- Acetamiprid-N-desmethyl
- Acetochlor
- Acetochlor ESA sodium salt
- Acetochlor OA
- Acetochlor SAA
- Acetone
- Acetyl glyphosate
- Acetyldeoxynivalenol
- Acetylsalicylic acid
- Acetylsulfamethoxazole
- Acibenzolar-S-methyl
- Acifluorfen
- Aclonifen
- Acrinathrin
- Acrolein-2,4-DNPH
- Acrylamide
- Afidopyropen
- AHMI (Phantolide)
- AHTN (Tonalid)
- Alachlor
- Alachlor ESA sodium salt
- Alachlor OA
- Albendazole
- Albendazole sulfoxide
- Albendazole-2-aminosulfone hydrochloride
- Aldicarb
- Aldicarb-sulfone
- Aldicarb-sulfoxide
- Aldrin
- Allethrin
- Alodane
- Altenuene
- Alternariol
- Alternariol monomethyl ether
- Ametoctradin
- Ametryn
- Amidithion
- Amidosulfuron
- Amino-6-chloro-1,3-benzenedisulfonamide
- Aminobiphenyl
- Aminocarb
- Aminoflubendazole
- Aminophenol
- Aminopyralid
- Aminopyridine
- Amisulbrom
- Amitraz
- Amitriptyline hydrochloride
- Amitrole
- AMOZ
- AMPA
- AMPPA
- Anilazine
- Aniline
- Anisidine
- Anthracene
- Anthraquinone
- Antipyrine
- Arprinocid
- Aspon
- Asulam
- Atenolol
- Atranol
- Atrazin
- Atrazine
- Atrazine-2-hydroxy
- Atrazine-desethyl
- Atrazine-desethyl-desisopropyl
- Atrazine-desisopropyl
- Atrazine-desisopropyl-2-hydroxy
- Atropine
- Avermectin B1a
- Avermectin B1b
- Avobenzone
- Azaconazole
- Azadirachtin A
- Azamethiphos
- Azaperol
- Azinphos-ethyl
- Azinphos-methyl
- Aziprotryne
- Azocyclotin
- Azoxystrobin
- Azoxystrobin (free acid)
- Azoxystrobin Metabolite R401553
- Azoxystrobin Metabolite R402173
- Azoxystrobin R230310
- B
- Bambuterol hydrochloride
- Baquiloprim
- Barban
- Beflubutamid
- Benalaxyl
- Bendiocarb
- Benfluralin
- Benfuracarb
- Benfuresate
- Benoxacor
- Bensulfuron-methyl
- Bensulide
- Bentazone
- Bentazone-6-hydroxy
- Bentazone-8-hydroxy
- Benthiavalicarb-isopropyl
- Benzaldehyde
- Benzene
- Benzidine
- Benzobicyclon
- Benzofenap
- Benzoic acid
- Benzophenone
- Benzotriazole
- Benzovindiflupyr
- Benzoximate
- Benzoylprop-ethyl
- Benzo[a]pyrene
- Benzo[b]fluoranthene
- Benzo[c]fluorene
- Benzo[e]pyrene
- Benzo[g,h,i]perylene
- Benzo[j]fluoranthene
- Benzo[k]fluoranthene
- Benzthiazuron
- Benzyl butyl phthalate
- Benzylalcohol
- Benzylaminopurine
- Benzyldimethyldecylammonium chloride
- Benzyldimethyldodecylammonium chloride
- Benzyldimethylhexadecylammonium chloride
- Benzyldimethyloctadecylammonium chloride
- Benz[a]anthracene
- Bezafibrate
- Bicyclopyrone
- Bifenazate
- Bifenazate-diazene
- Bifenox
- Bifenthrin
- Binapacryl
- Biphenyl
- Bis(2-ethylhexyl) adipate
- Bis(2-ethylhexyl) phthalate
- Bis(methylglycol) phthalate
- Bis-palmitoyl-3-chloropropanediol
- Bisphenol A
- Bisphenol S
- Bitertanol
- Bixafen
- Boldenone
- Boscalid (Nicobifen)
- Boscalid Metabolite M510F01
- Brodifacoum
- Broflanilide
- Bromacil
- Bromadiolone
- Brombuterol hydrochloride
- Bromfenvinphos-ethyl
- Bromoacetic acid
- Bromoaniline
- Bromocyclen
- Bromofluorobenzene
- Bromoform
- Bromophenylurea
- Bromophos-ethyl
- Bromophos-methyl
- Bromopropylate
- Bromoxynil
- Bromoxynil-methyl ether
- Bromoxynil-octanoate
- Bromuconazole
- Bufencarb
- Bupirimate
- Buprofezin
- Butachlor
- Butafenacil
- Butamifos
- Butanol
- Butocarboxim
- Butocarboxim-sulfoxide
- Butoxycarboxim
- Butralin
- Buturon
- Butyl Ethyl Ether
- Butyl methyl ether
- Butyl phenyl ether
- Butylate
- Butylated hydroxyanisole
- Butylbenzene
- Butylparaben
- Butylphenol
- C
- Cadusafos
- Caffeine
- Cambendazole
- Camphechlor (Toxaphene)
- Camphor
- Cannabidiolic acid
- Captafol
- Captan
- Carazolol
- Carbamazepine
- Carbamazepine 10,11-epoxide
- Carbaryl
- Carbendazim
- Carbetamide
- Carbofuran
- Carbofuran-3-hydroxy
- Carbofuran-3-keto
- Carbon disulfide
- Carbophenothion
- Carbophenothion-methyl
- Carbophenothion-methyl-sulfone
- Carbosulfan
- Carboxin
- Carboxin-sulfoxide
- Carbuterol acetate hydrate
- Carfentrazone (free acid)
- Carfentrazone-ethyl
- Carnidazole
- Carpropamid
- CDPOS
- Cephacetrile
- Chinomethionat
- Chloramphenicol
- Chlorantraniliprole
- Chlorbenside
- Chlorbenside-sulfone
- Chlorbromuron
- Chlorbufam
- Chlordane
- Chlordecone (Kepone)
- Chlordecone alcohol
- Chlordimeform
- Chlorethoxyfos
- Chlorfenapyr
- Chlorfenprop-methyl
- Chlorfenson
- Chlorfenvinphos
- Chlorfluazuron
- Chlorflurenol-methyl ester
- Chloridazon
- Chloridazon-desphenyl
- Chloridazon-methyl-desphenyl
- Chlorimuron-ethyl
- Chlormadinone acetate
- Chlormephos
- Chlormequat chloride
- Chloro-1,2-propanediol
- Chloro-2-methylaniline
- Chloro-2-nitroaniline
- Chloro-3-methylphenol
- Chloroacetic Acid
- Chloroaniline
- Chloroatranol
- Chlorobenzene
- Chlorobenzilate
- Chlorobenzoic acid
- Chlorobenzuron
- Chlorobutane
- Chloroethanol
- Chloroethyl linoleate
- Chloroethyl oleate
- Chloronaphthalene
- Chloroneb
- Chloronicotinic acid
- Chloronitrobenzene
- Chlorophacinone
- Chlorophenol
- Chlorophenoxyacetic acid
- Chloropropylate
- Chlorothalonil
- Chlorothalonil-4-hydroxy
- Chlorotoluene
- Chlorotoluron
- Chlorotoluron-desmethyl
- Chloroxuron
- Chlorpromazine hydrochloride
- Chlorpropham
- Chlorpyrifos
- Chlorpyrifos-methyl
- Chlorpyrifos-methyl-desmethyl TMA salt
- Chlorsulfuron
- Chlortetracycline hydrochloride
- Chlorthal-dimethyl
- Chlorthalidone
- Chlorthiamid
- Chlorthion
- Chlorthiophos
- Chlozolinate
- Cholecalciferol
- Chromafenozide
- Chrysene
- Cimaterol
- Cimbuterol
- Cinidon-ethyl
- Cinmethylin
- Cinosulfuron
- Ciprofloxacin hydrochloride hydrate
- Citronellol
- Clenbuterol hydrochloride
- Clenpenterol hydrochloride
- Clenproperol
- Clethodim
- Clethodim-sulfone
- Climbazole
- Clindamycin hydrochloride
- Clodinafop (free acid)
- Clodinafop-propargyl
- Clofentezine
- Clofibrate
- Clofibric acid
- Clomazone
- Clomeprop
- Clopyralid
- Cloquintocet-mexyl
- Closantel
- Clothianidin
- Clotrimazole
- Clozapine
- Colchicin
- Cotinine
- Coumachlor
- Coumaphos
- Coumarin
- Crimidine
- Crotoxyphos
- Cyanazine
- Cyanofenphos
- Cyanophos
- Cyantraniliprole
- Cyanuric acid
- Cyazofamid
- Cyclanilide
- Cyclaniliprole
- Cycloate
- Cyclopenta[c,d]pyrene
- Cycloxydim
- Cycloxydim Metabolite BH 517-TGSO2
- Cycluron
- Cyflufenamid
- Cyflumetofen
- Cyfluthrin
- Cyhalofop-butyl
- Cyhalothrin
- Cyhexatin
- Cymiazole
- Cymoxanil
- Cypermethrin
- Cyphenothrin
- Cyproconazole
- Cyprodinil
- Cyprosulfamide
- Cyromazine
- D
- D methyl ester
- D5-Glycidyl palmitate
- Daimuron
- Dalapon
- Dazomet
- DB
- DB-methyl ester
- DDD
- DDE
- DDT
- Decane
- Decanol
- DEET
- Deltamethrin
- Demeton-O
- Demeton-S
- Demeton-S-methyl
- Demeton-S-methyl-sulfone
- Demeton-S-methyl-sulfoxide
- Deoxynivalenol
- Desmedipham
- Desmetryn
- Desoxy-Mequindox
- Desoxycarbadox
- Di-n-octyl phthalate
- Di-n-propyltin dichloride
- Di-tert-butyl-4-methylphenol
- Di-tert-butylphenol
- Diacetylbenzene
- Diafenthiuron
- Dialifos
- Diallate
- Diaminoanisole
- Diaminotoluene
- Diazinon
- Dibenzo[a,e]pyrene
- Dibenzo[a,h]pyrene
- Dibenzo[a,i]pyrene
- Dibenzo[a,l]pyrene
- Dibenz[a,h]anthracene
- Dibromobenzophenone
- Dibromophenol
- Dibutyl phthalate
- Dicamba
- Dicamba-desmethyl
- Dicamba-methyl ester
- Dichlobenil
- Dichlofenthion
- Dichlofluanid
- Dichlone
- Dichlormid
- Dichloroacetic acid
- Dichloroaniline
- Dichlorobenzamide
- Dichlorobenzene
- Dichlorobenzidine
- Dichlorobenzoic Acid
- Dichlorobenzophenone
- Dichlorobenzyl chloride
- Dichloroethane
- Dichloroethene
- Dichloroisoeverninic acid
- Dichloronitrobenzene
- Dichlorophenol
- Dichlorophenyl)-3-methylurea
- Dichlorophenyl)urea
- Dichloropropan-2-ol
- Dichloropropane
- Dichlorotoluene
- Dichlorovinyl)-2,2-dimethylcyclopropane-1-carboxylic acid
- Dichlorprop
- Dichlorprop-P
- Dichlorvos
- Diclobutrazol
- Diclofenac sodium
- Diclofop (free acid)
- Diclofop-methyl
- Dicloran
- Dicofol
- Dicrotophos
- Dicyandiamide
- Dicyclanil
- Dicyclohexyl phthalate
- Dieldrin
- Diethanolamine
- Diethofencarb
- Diethyl phthalate
- Diethylbenzene
- Diethylene glycol dimethyl ether
- Diethylstilbestrol (mixture of Isomers)
- Difenacoum
- Difenoconazole
- Difenoxuron
- Diflubenzuron
- Diflufenican
- Diflufenzopyr
- Difluoroacetic acid
- Difluorophenylacetic acid
- Dihexyl phthalate
- Dihydroxybenzoic acid
- Dihydroxybenzophenone
- Diisobutyl phthalate
- Diisodecyl phthalate
- Diisononyl phthalate
- Diisopentyl Phthalate
- Diisopropylbenzene
- Dikegulac sodium
- Diltiazem hydrochloride
- Dimefox
- Dimefuron
- Dimethachlor
- Dimethachlor ESA sodium salt
- Dimethachlor Metabolite CGA 369873
- Dimethachlor OA
- Dimethenamid
- Dimethenamid ESA sodium salt
- Dimethenamid OA
- Dimethenamid-P
- Dimethipin
- Dimethoate
- Dimethomorph
- Dimethoxybenzoic acid
- Dimethyl fumarate
- Dimethyl phthalate
- Dimethylaniline
- Dimethyldioctadecylammonium chloride
- Dimethylformamide
- Dimethylnaphthalene
- Dimethylpentane
- Dimethylphenol
- Dimethylphenyl)formamide
- Dimethylphenyl-N-methylformamidine HCl
- Dimethylsulfamide
- Dimethylvinphos
- Dimetilan
- Dimetridazole
- Dimetridazole-2-hydroxy
- Dimoxystrobin
- Diniconazole
- Dinitolmide
- Dinitrobenzene
- Dinitrophenol
- Dinitrosalicylic acid
- Dinitrotoluene
- Dinobuton
- Dinocap
- Dinoseb
- Dinotefuran
- Dinoterb
- Dioxacarb
- Dioxane
- Dioxathion
- Diphenamid
- Diphenylamine
- Dipropetryn
- Dipropyl phthalate
- Diquat dibromide
- Diquat dibromide monohydrate
- Disulfoton
- Disulfoton-sulfone
- Disulfoton-sulfoxide
- Ditalimfos
- Dithianon
- Dithiopyr
- Diuron
- DMSA
- DMST
- DNOC
- Dodemorph
- Dodine
- DPOSA
- E
- Edifenphos
- Eicosane
- Emamectin B1a
- Emamectin B1b
- Emamectin benzoate
- Endosulfan
- Endosulfan-sulfate
- Endrin
- Endrin ketone
- Epichlorohydrin
- Epichlortetracycline hydrochloride
- EPN
- Epoxiconazole
- EPTC
- Ergocalciferol
- Erlose
- Erythromycin
- Esfenvalerate
- Estradiol
- Estrone
- Etaconazole
- Ethalfluralin
- Ethanol
- Ethanolamine
- Ethephon
- Ethidimuron
- Ethiofencarb
- Ethiofencarb-sulfone
- Ethiofencarb-sulfoxide
- Ethion
- Ethiprole
- Ethirimol
- Ethofumesate
- Ethofumesate-2-keto
- Ethoprophos
- Ethoxyquin
- Ethoxyquin Dimer
- Ethyl-N-(2-hydroxyethyl)perfluorooctylsulfonamide
- Ethylaniline
- Ethylbenzene
- Ethylene thiourea
- Ethylguaiacol
- Ethylisopropylnitrosamine
- Ethylparaben
- Ethylphenol
- Ethyltoluene
- Ethynylestradiol
- Etofenprox
- Etoxazole
- Etridiazole
- Etrimfos
- Eugenol
- F
- Famoxadone
- Famphur
- Febantel
- Fenamidone
- Fenamiphos
- Fenamiphos-sulfone
- Fenamiphos-sulfoxide
- Fenarimol
- Fenazaquin
- Fenbendazole
- Fenbuconazole
- Fenbutatin oxide
- Fenchlorazole-ethyl
- Fenchlorphos
- Fenchlorphos-oxon
- Fenfluthrin
- Fenfuram
- Fenhexamid
- Fenitrothion
- Fenobucarb
- Fenofibrate
- Fenofibric acid
- Fenoprop
- Fenothiocarb
- Fenoxanil
- Fenoxaprop-P
- Fenoxaprop-P-ethyl
- Fenoxycarb
- Fenpiclonil
- Fenpropathrin
- Fenpropidin
- Fenpropimorph
- Fenpyrazamine
- Fenpyroximate
- Fenson
- Fensulfothion
- Fensulfothion-oxon
- Fensulfothion-oxon-sulfone
- Fensulfothion-sulfone
- Fenthion
- Fenthion-oxon
- Fenthion-oxon-sulfone
- Fenthion-oxon-sulfoxide
- Fenthion-sulfone
- Fenthion-sulfoxide
- Fentin acetate
- Fentin chloride
- Fenuron
- Fenvalerate
- Ferimzone
- Fipronil
- Fipronil-desulfinyl
- Fipronil-sulfide
- Fipronil-sulfone
- Firocoxib
- Flamprop
- Flamprop-methyl
- Flazasulfuron
- Flonicamid
- Florasulam
- Florfenicol amine
- Florpyrauxifen-benzyl
- Fluacrypyrim
- Fluazifop
- Fluazifop-butyl
- Fluazifop-methyl
- Fluazifop-P-butyl
- Fluazinam
- Fluazuron
- Flubendazole
- Flubendiamide
- Flubenzimine
- Fluchloralin
- Flucycloxuron
- Flucythrinate
- Fludioxonil
- Fluensulfone
- Flufenacet
- Flufenacet ESA sodium salt
- Flufenacet Metabolite FOE5043
- Flufenoxuron
- Flufenzine
- Flumequine
- Flumethrin
- Flumetralin
- Flumetsulam
- Flumioxazin
- Flunixin
- Fluometuron
- Fluopicolide
- Fluopyram
- Fluoranthene
- Fluorene
- Fluoroaniline
- Fluoroglycofen-ethyl
- Fluotrimazole
- Fluoxastrobin
- Flupyradifurone
- Flupyrimin
- Flupyrsulfuron-methyl
- Fluquinconazole
- Flurenol-butyl ester
- Flurochloridone
- Fluroxypyr
- Fluroxypyr-1-methylheptylester
- Flurprimidol
- Flurtamone
- Flusilazole
- Fluthiacet-methyl
- Flutianil
- Flutolanil
- Flutriafol
- Fluvalinate
- Fluvoxamine maleate
- Fluxametamide
- Fluxapyroxad
- Folpet
- Fomesafen
- Fonofos
- Foramsulfuron
- Forchlorfenuron
- Formaldehyde-2,4-DNPH
- Formetanate hydrochloride
- Formothion
- Fosetyl-aluminium
- Fosthiazate
- FRD-902
- FRD-903
- Fructose
- Fuberidazole
- Furalaxyl
- Furametpyr
- Furan
- Furathiocarb
- Furazolidone
- Furosemide
- G
- Gabapentin
- Galactosan
- Gemfibrozil
- Genite
- Geraniol
- Glucoheptose
- Glucose
- Glufosinate hydrochloride
- Glufosinate-ammonium
- Glufosinate-N-acetyl
- Glutaraldehyde-DNPH
- Glycidyl palmitate
- Glycidyl Stearate
- Glyphosate
- Glyphosate isopropylamine salt
- H
- Halauxifen (free acid)
- Halauxifen-methyl
- Halfenprox
- Halofenozide
- Halofuginone hydrobromide
- Halosulfuron-methyl
- Haloxyfop
- Haloxyfop-2-ethoxyethyl
- Haloxyfop-methyl
- HCH
- Heptachlor
- Heptachlor-endo-epoxide
- Heptachlor-exo-epoxide
- Heptachlorodecane (CP-8)
- Heptadecanoic acid-methyl ester
- Heptafluorobutyric acid
- Heptenophos
- Heptyltintrichloride
- Hexabromocyclododecane
- Hexachloro-1,3-butadiene
- Hexachlorobenzene
- Hexaconazole
- Hexaflumuron
- Hexazinone
- Hexythiazox
- HHCB (Galaxolide)
- Higenamine hydrochloride
- Homovanillic acid
- Hydramethylnon
- Hydrochlorothiazide
- Hydroprene
- Hydroxy-propoxycarbazone
- Hydroxybenzonitrile
- Hydroxyclomazone
- Hydroxyflunixin
- Hydroxyibuprofen
- Hydroxymebendazole
- Hydroxymethyl)-2-furaldehyd
- Hydroxymethylclenbuterol hydrochloride
- Hydroxyproline
- Hydroxystanozolol
- Hydroxythiabendazole
- I
- Ibuprofen
- Imazalil
- Imazamethabenz (free acid)
- Imazamethabenz-methyl
- Imazamox
- Imazapic
- Imazapyr
- Imazaquin
- Imazethapyr
- Imazosulfuron
- Imibenconazole
- Imidacloprid
- Imidacloprid guanidine hydrochloride
- Imidacloprid olefin
- Indaziflam
- Indeno[1,2,3-c,d]pyrene
- Indoxacarb
- Iodofenphos
- Iodosulfuron-methyl sodium
- Iohexol
- Iopromide
- Ioxynil
- Ioxynil-methyl
- Ioxynil-octanoate
- Ipconazole
- IPPD-Quinone
- Iprobenfos
- Iprodione
- Ipronidazole
- Ipronidazole-hydroxy
- Iprovalicarb
- Irbesartan
- Isazofos
- Isobutyl-3-methoxypyrazine
- Isocarbophos
- Isocycloseram
- Isodrin
- Isofenphos
- Isofenphos-methyl
- Isofenphos-oxon
- Isofetamid
- Isomethiozin
- Isonoruron
- Isoprocarb
- Isopropalin
- Isopropyl-3-methoxypyrazine
- Isopropyl-6-methyl-4-pyrimidinol
- Isopropylaniline
- Isopropylparaben
- Isoprothiolane
- Isoproturon
- Isopyrazam
- Isoxaben
- Isoxadifen-ethyl
- Isoxaflutole
- Isoxaflutole diketonitrile RPA 202248
- Isoxathion
- Ivermectin
- J
- K
- L
- Labetalol hydrochloride
- Lasalocid A sodium salt
- Lenacil
- Lepimectin
- Leptophos
- Lidocaine
- Limonene
- Linalool
- Linuron
- Lomefloxacin hydrochloride
- Lufenuron
- M
- Mabuterol hydrochloride
- Malaoxon
- Malathion
- Maleic hydrazide
- Malic acid
- Maltotetraose
- Mandestrobin
- Mandipropamid
- Mapenterol hydrochloride
- Matrine
- MCPA
- MCPB
- MCPD sodium sulfate
- Mebendazole
- Mecarbam
- Mecoprop
- Mecoprop-1-octyl ester
- Medroxyprogesterone
- Mefenacet
- Mefenamic acid
- Mefenpyr-diethyl
- Mefentrifluconazole
- Megestrol
- Melamine
- Meloxicam
- Menthol
- Mepanipyrim
- Mepanipyrim-2-hydroxypropyl
- Mepiquat iodide
- Mepivacaine hydrochloride
- Mepronil
- Meptyldinocap
- Mercaptobenzimidazole
- Mesosulfuron-methyl
- Mesotrione
- Metaflumizone
- Metalaxyl
- Metalaxyl Metabolite CGA 108906
- Metalaxyl-M
- Metamitron
- Metazachlor
- Metazachlor ESA
- Metazachlor ESA sodium salt
- Metazachlor Metabolite 479M16
- Metazachlor OA
- Metconazole
- Metformin hydrochloride
- Methabenzthiazuron
- Methacrifos
- Methacrolein-2,4-DNPH
- Methamidophos
- Methanol
- Methidathion
- Methimazole
- Methiocarb
- Methiocarb-sulfone
- Methiocarb-sulfoxide
- Methomyl
- Methoprene
- Methoprotryne
- Methoxyacetophenone
- Methoxychlor
- Methoxyfenozide
- Methoxymethylaniline
- Methyl salicylate
- Methyl-1H-benzotriazole
- Methyl-4-nitroso-piperazine
- Methylchrysen
- Methylchrysene
- Methylhippuric acid
- Methylimidazole
- Methylnaphthalene
- Methylparaben
- Methylpentane
- Methylperfluorooctane sulfonamidoacetic acid
- Methylphenol
- Methylphosphinico)propionic acid
- Methylsulfonyl)-2-nitrobenzoic acid
- Metobromuron
- Metolachlor
- Metolachlor ESA sodium salt
- Metolachlor Metabolite CGA 368208
- Metolachlor Metabolite CGA 37735
- Metolachlor Metabolite NOA 413173
- Metolachlor OA
- Metolcarb
- Metominostrobin
- Metoprolol tartrate
- Metosulam
- Metoxuron
- Metoxuron-monomethyl
- Metrafenone
- Metribuzin
- Metribuzin-desamino
- Metronidazole
- Metronidazole-hydroxy
- Metsulfuron-methyl
- Metyltetraprole
- Mevinphos
- Mexacarbate
- Milbemectin A3
- Milbemectin A4
- Miloxacin
- Mirex
- Molinate
- Monocrotophos
- Monolinuron
- Monononadecanoin
- Monuron
- Morpholine
- Morphothion
- Moxidectin
- Musk ketone
- Musk xylene
- Myclobutanil
- N
- Naled
- Nalidixic acid
- Naphthalene
- Naphthol
- Naphthoxyacetic acid
- Naphthylamine
- Napropamide
- Naproxen
- Naptalam
- Narasin
- Naringenin
- Neburon
- Nicotine
- Nifursol-desfurfuryliden
- Nitenpyram
- Nitralin
- Nitrapyrin
- Nitro-benzaldehydesemicarbazone
- Nitrobenzene
- Nitrofen
- Nitrofurantoin
- Nitrofurazone
- Nitrophenol
- Nitrophenyl a-D-maltohexaoside
- Nitropropane
- Nitroso-di-n-butylamine
- Nitroso-N-methyl-4-aminobutyric acid
- Nitroso-N-methylaniline
- Nitrosobis(2-hydroxyethyl)amine
- Nitrosodibenzylamine
- Nitrosodibutylamine
- Nitrosodiethylamine
- Nitrosodiisobutylamine
- Nitrosodiisopropylamine
- Nitrosodimethylamine
- Nitrosodipropylamine
- Nitrosoethylmethylamine
- Nitrosomorpholine
- Nitrosopiperidine
- Nitrosopyrrolidine
- Nitrothal-isopropyl
- Nitrotoluene
- NMP
- Nonachlor
- Nonafluoro-1-butanesulfonic acid
- Nonafluoropentanoic acid
- Nonylphenol
- Nonylphenol (technical mixture)
- Nonylphenol-di-ethoxylate
- Nonylphenol-mono-ethoxylate
- Norepinephrine hydrochloride
- Norfloxacin
- Norflurazon
- Novaluron
- NP-AHD
- NP-AMOZ
- NP-DNSAH
- Nuarimol
- O
- Ochratoxin A
- Ochratoxin alpha
- Octachlorostyrene
- Octane
- Octanediol
- Octanoic acid 4-cyano-2,6-diiodophenyl ester
- Octanol
- Octocrylene
- Octylphenol
- Octylphenol-di-ethoxylate
- Octylphenol-mono-ethoxylate
- Ofloxacin
- Ofurace
- Oleoyl Ethanolamide
- Omethoate
- Orciprenaline acetate hydrate
- Oryzalin
- Oxadiargyl
- Oxadiazon
- Oxadixyl
- Oxamyl
- Oxamyl-oxime
- Oxasulfuron
- Oxathiapiprolin
- Oxfendazole
- Oxibendazole
- Oxolinic acid
- Oxybenzone
- Oxycarboxin
- Oxyclozanide
- Oxyfluorfen
- Oxytetracycline hydrochloride
- P
- Paclobutrazol
- PAH-Mix 9
- Paraoxon-ethyl
- Paraoxon-methyl
- Paraquat dichloride
- Parathion-ethyl
- Parathion-methyl
- Patulin
- Pazufloxacin mesylate
- PCB 116
- PCB 180
- PCB 209
- PCB 52
- Pebulate
- Penconazole
- Pencycuron
- Pencycuron-PB-amine
- Pendimethalin
- Penflufen
- Penicillin V potassium salt
- Penoxsulam
- Pentachloroaniline
- Pentachloroanisole
- Pentachlorobenzene
- Pentachlorophenol
- Pentachlorothioanisole
- Pentadecafluorooctanoic acid
- Pentaerythrityltetranitrate
- Penthiopyrad
- Pentyl-iso-pentylphthalate
- Perfluorodecanesulfonic acid
- Perfluorohexanesulfonic acid
- Perfluorononanesulfonic acid sodium salt
- Perfluorooctanoic acid
- Perfluorotetradecanoic acid
- Permethrin
- Perthane
- Perylene
- Pethoxamid
- Phenanthrene
- Phenetidine
- Phenkapton
- Phenmedipham
- Phenol
- Phenothrin
- Phenoxyethanol
- Phenthoate
- Phenyl-2-thiouracil
- Phenylacetaldehyde (stabilized)
- Phenylbutazone
- Phenylenediamine
- Phenylethanolamine A
- Phenylphenol
- Phorate
- Phorate-oxon
- Phorate-oxon-sulfone
- Phorate-oxon-sulfoxide
- Phorate-sulfone
- Phorate-sulfoxide
- Phosalone
- Phosfolan
- Phosmet
- Phosmet-oxon
- Phosphamidon
- Phosphonic acid
- Phoxim
- Phthalimide
- Picaridin
- Picloram
- Picolinafen
- Picoline
- Picoxystrobin
- Pinene
- Pinoxaden
- Piperonyl butoxide
- Pirimicarb
- Pirimicarb-desamido-desmethyl
- Pirimicarb-desmethyl
- Pirimicarb-desmethyl-formamido
- Pirimiphos-ethyl
- Pirimiphos-methyl
- Pirimiphos-methyl-desmethyl TMA salt
- Piroxicam
- Plifenate
- Potasan
- PPD
- PPD-quinone
- Prallethrin
- Pretilachlor
- Primidone
- Primisulfuron-methyl
- Prochloraz
- Prochloraz desimidazole-amino BTS44595
- Prochloraz Metabolite BTS40348
- Prochloraz metabolite BTS44596
- Procymidone
- Profenofos
- Profluralin
- Profoxydim lithium salt
- Progesterone
- Promecarb
- Prometon
- Prometryn
- Propachlor
- Propachlor ESA sodium salt
- Propachlor OA
- Propamocarb
- Propamocarb hydrochloride
- Propanil
- Propaquizafop
- Propargite
- Propazine
- Propetamphos
- Propham
- Propiconazole
- Propisochlor
- Propoxur
- Propoxycarbazone sodium
- Propranolol hydrochloride
- Propylene glycol
- Propylene thiourea
- Propylparaben
- Propyltin chloride
- Propyzamide
- Proquinazid
- Prosulfocarb
- Prosulfuron
- Prothioconazole
- Prothioconazole-desthio
- Prothiofos
- Prothoate
- Pydiflumetofen
- Pymetrozine
- Pyracarbolid
- Pyraclofos
- Pyraclostrobin
- Pyraflufen monohydrate (free acid)
- Pyraflufen-ethyl
- Pyrasulfotole
- Pyrazophos
- Pyrazoxyfen
- Pyrene
- Pyrethrins
- Pyribencarb
- Pyridaben
- Pyridafol
- Pyridalyl
- Pyridaphenthion
- Pyridate
- Pyrifenox
- Pyrimethanil
- Pyrimethanil Metabolite M605F002
- Pyrimidifen
- Pyriminobac-methyl
- Pyriofenone
- Pyriproxyfen
- Pyroxasulfone
- Q
- QDI
- Quinalphos
- Quinclorac
- Quinmerac
- Quinoclamine
- Quinoline
- Quinoxyfen
- Quintiofos
- Quintozene
- Quizalofop
- Quizalofop-ethyl
- Quizalofop-P-ethyl
- Quizalofop-p-tefuryl
- R
- S
- S421
- Saccharin
- Saccharose
- Saflufenacil
- Saflufenacil Metabolite M800H11
- Saflufenacil Metabolite M800H35
- Salbutamol acetate
- Salbutamol hemisulfate salt
- Salicylic acid
- Salmeterol xinafoate
- Sarafloxacin hydrochloride
- Scopolamine hydrobromide trihydrate
- Sebuthylazine
- Secbumeton
- Secnidazole
- Sedaxane
- Semduramicin sodium
- Sethoxydim
- Siduron
- Silafluofen
- Silthiofam
- Simazine
- Simazine-2-hydroxy
- Simvastatin
- Sisapronil
- Sodium chlorate
- Sodium cyclamate
- Sodium perchlorate
- Sodium perchlorate monohydrate
- Sorbitol
- Sotalol hydrochloride
- Spinetoram
- Spinosad
- Spinosyn D
- Spirodiclofen
- Spiromesifen
- Spirotetramat
- Spirotetramat-enol-glucoside
- Spirotetramat-keto-hydroxy
- Spirotetramat-mono-hydroxy
- Spiroxamine
- Stanozolol
- Styrene
- Sulcotrione
- Sulfacetamide
- Sulfachloropyridazine
- Sulfadiazine
- Sulfadimethoxine
- Sulfadoxine
- Sulfaguanidine monohydrate
- Sulfamerazine
- Sulfamethazine
- sulfamethizole
- Sulfamethoxazole
- sulfamethoxypyridazine
- Sulfamonomethoxine
- Sulfamoxole
- Sulfanilamide
- Sulfanitran
- Sulfaphenazole
- Sulfapyridine
- Sulfaquinoxaline sodium salt
- Sulfathiazole
- Sulfentrazone
- Sulfisoxazole
- Sulfosulfuron
- Sulfotep
- Sulfoxaflor
- Sulprofos
- Sulprofos-sulfoxide
- Surrogate Mix ISO 27065 and ISO 18889
- Swep
- T
- T-methyl ester
- Taleranol
- TCMTB (Busan)
- Tebuconazole
- Tebuconazole-tert-butyl-hydroxy
- Tebufenozide
- Tebufenpyrad
- Tebupirimfos
- Tebutam
- Tebuthiuron
- Tecnazene
- Teflubenzuron
- Tefluthrin
- Telodrin
- Tembotrione
- Tembotrione metabolite AE 1417268
- Temephos
- Tenuazonic acid mixture of diastereomers
- TEPP
- Tepraloxydim
- Terbacil
- Terbufos
- Terbufos-sulfone
- Terbufos-sulfoxide
- Terbumeton
- Terbumeton-desethyl
- Terbuthylazine
- Terbuthylazine-2-hydroxy
- Terbuthylazine-desethyl
- Terbuthylazine-desethyl-2-hydroxy
- Terbutryn
- Terphenyl
- Terpinene
- Terpineol
- Tetra-n-butyltin
- Tetrachloroanisole
- Tetrachlorobenzene
- Tetrachloroethane
- Tetrachloroethene
- Tetrachlorophenol
- Tetrachlorotoluene
- Tetrachlorvinphos
- Tetraconazole
- Tetradifon
- Tetrahydrophthalimide
- Tetramethrin
- Tetraniliprole
- Tetrasul
- TFNA
- TFNA-AM
- TFNG
- Thenylchlor
- Thiabendazole
- Thiacloprid
- Thiadone (Flufenacet metabolite)
- Thiamethoxam
- Thiamethoxam metabolite CGA 353968
- Thiazafluron
- Thidiazuron
- Thiencarbazone-methyl
- Thifensulfuron-methyl
- Thiobencarb
- Thiodicarb
- Thiofanox
- Thiofanox-sulfone
- Thiofanox-sulfoxide
- Thiometon
- Thionazin
- Thiophanate-ethyl
- Thiophanate-methyl
- Thiram
- Thymol
- Tiocarbazil
- Tolclofos-methyl
- Tolfenamic acid
- Tolfenpyrad
- Toltrazuril sulfone
- Toltrazuril-sulfoxide
- Toluene
- Toluic acid
- Toluidine
- Tolylfluanid
- Topramezone
- Tralkoxydim
- Tralomethrin
- Transfluthrin
- Trenbolone
- Triadimefon
- Triadimenol
- Triallate
- Triamiphos
- Triasulfuron
- Triazamate
- Triazophos
- Triazoxide
- Tribenuron-methyl
- Tribromoanisole
- Tribromophenol
- Tributyl phosphate
- Tributyltin chloride
- Trichlorfon
- Trichloroacetic acid
- Trichloroaniline
- Trichloroanisole
- Trichlorobenzene
- Trichloroethane
- Trichloroethene
- Trichloromethane
- Trichloronate
- Trichlorophenol
- Trichlorophenoxyacetic Acid
- Trichlorotoluene
- Trichodiene
- Triclabendazole
- Triclabendazole-sulfone
- Triclabendazole-sulfoxide
- Triclocarban
- Triclopyr
- Triclosan
- Triclosan-methyl
- Tricyclazole
- Tricyclohexyltin chloride
- Tridemorph (technical mixture)
- Trietazine
- Triethanolamine
- Triethyl phosphate
- Trifloxystrobin
- Triflumizole
- Triflumizole Metabolite FM-1-1
- Triflumizole Metabolite FM-6-1
- Triflumuron
- Trifluoroacetic acid sodium salt
- Trifluorophenylacetic acid
- Trifluralin
- Triflusulfuron-methyl
- Triforine
- Trimethacarb
- Trimethoprim
- Trimethoxyphenylacetic acid
- Trimethylaniline
- Trimethylbenzene
- Trimethylphenol
- Trimethylsulfonium iodide
- Trimetoquinol hydrochloride
- Trinexapac
- Trinexapac-ethyl
- Trinitrotoluene
- Triphenylphosphate
- Triticonazole
- Tritosulfuron
- U
- V
- Valifenalate
- Valsartan
- Vamidothion
- Vamidothion-sulfone
- Vamidothion-sulfoxide
- Vanillin
- Venlafaxine hydrochloride
- Versandkosten
- Vinclozolin
- X
- Z
NP-AMOZ
Ensure Accurate Residue Analysis with High-Purity NP-AMOZ Reference Materials
NP-AMOZ, a metabolite of the veterinary antibiotic furaltadone, is essential for laboratories conducting residue analysis in food and environmental samples. HPC Standards GmbH offers high-purity NP-AMOZ reference materials, tested to meet international quality requirements and the highest industrial standards. These materials are crucial for calibrating instruments, validating analytical methods, and ensuring compliance with regulatory limits set by bodies like the EU and FDA. Protect public health and ecosystems with reliable NP-AMOZ monitoring.
Product | Catalog No./ CAS No. | Quantity | Price | |
|---|---|---|---|---|
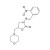 | 679153 | 1X10MG | Please log in. | |
2-NP-AMOZ solution | 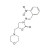 | 689507 | 1X1ML | Please log in. |
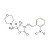 | 679154 | 1X10MG | Please log in. | |
D5-2-NP-AMOZ solution | 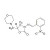 | 690758 | 1X1ML | Please log in. |
High-Purity Reference Materials for Accurate Residue Analysis
Overview
NP-AMOZ is a metabolite of the antibiotic furaltadone, which is used in veterinary medicine. It is crucial for laboratories to have high-purity reference materials for NP-AMOZ to ensure accurate residue analysis in food and environmental samples.
Uses
NP-AMOZ is primarily used in residue analysis to monitor and ensure that food products comply with regulatory limits for veterinary drug residues. Laboratories use reference materials to calibrate their instruments and validate their analytical methods.
Regulatory
Regulatory bodies such as the European Union (EU) and the United States Food and Drug Administration (FDA) have set maximum residue limits (MRLs) for NP-AMOZ in food products. Compliance with these regulations is essential to ensure food safety and public health.
Monitoring
Regular monitoring of NP-AMOZ residues in food and environmental samples is necessary to ensure compliance with regulatory standards. Laboratories rely on high-purity reference materials to achieve accurate and reliable results.
Health Impact
Human Toxicity
Exposure to NP-AMOZ can pose health risks to humans, including potential carcinogenic effects. Accurate residue analysis helps in assessing and mitigating these risks.
Environmental Impact
Effects on Wildlife
NP-AMOZ residues can also impact wildlife, particularly aquatic organisms, when they enter the environment through agricultural runoff. Monitoring and controlling these residues is essential to protect ecosystems.
Safety Measures
Proper handling and disposal of NP-AMOZ and its reference materials are crucial to prevent contamination and exposure. Laboratories must follow safety guidelines and use appropriate protective equipment.
Regulation
Regulatory frameworks for NP-AMOZ are established by international bodies to ensure that food products are safe for consumption. Compliance with these regulations is mandatory for food producers and laboratories.
Analytical Standards
High-purity reference materials for NP-AMOZ provided by HPC Standards GmbH are tested according to international quality requirements and meet the highest industrial standards. These reference materials are essential for accurate calibration and validation of analytical methods in laboratories.


