New user? / Forgot your password?
0 Item | 0,00 €
Clozapine Reference Materials
Clozapine Reference Materials for Precise Residue Analysis
Unlock the highest standards in residue analysis with our high-purity Clozapine reference materials. Essential for laboratories focused on food and environmental analysis, our products ensure compliance with stringent regulatory limits. Trust HPC Standards GmbH for reliable and accurate results.
Product | Catalog No./ CAS No. | Quantity | Price | |
|---|---|---|---|---|
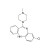 | 690350 | 1X100MG | Please log in. | |
D4-Clozapine solution | 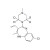 | 684072 | 1X1ML |
High-quality reference materials for accurate and reliable residue analysis of Clozapine.
Overview
Clozapine is a psychiatric medication and the first atypical antipsychotic discovered. It is primarily used to treat schizophrenia and schizoaffective disorder in patients who have not responded adequately to other antipsychotics. It is also used for treating psychosis in Parkinson's disease.
Uses
Clozapine is recommended for patients with schizophrenia or schizoaffective disorder who have not responded to two other antipsychotics. It is also used to treat psychosis in Parkinson's disease and is the only antipsychotic known to reduce the risk of suicide in these patients.
Regulatory
Clozapine is regulated as a prescription-only medication in many countries. In the US, it has a black box warning for several severe side effects, including agranulocytosis and myocarditis. It is listed on the World Health Organization's List of Essential Medicines.
Monitoring
Patients prescribed clozapine require regular blood monitoring due to the risk of agranulocytosis. Full blood counts are typically performed weekly for the first 18 weeks, then less frequently thereafter. Monitoring of other parameters such as weight, blood pressure, and markers for myocarditis is also recommended.
Health Impact
Clozapine has a range of side effects, including drowsiness, constipation, hypersalivation, tachycardia, and weight gain. It is not commonly associated with tardive dyskinesia but can cause agranulocytosis, seizures, myocarditis, and hyperglycemia.
Human Toxicity
Potential adverse effects include agranulocytosis, seizures, myocarditis, and hyperglycemia. The use of clozapine can rarely result in clozapine-induced gastric hypomotility syndrome, which may lead to bowel obstruction and death.
Environmental Impact
The environmental impact of clozapine includes its effects on wildlife, particularly through contamination of water sources. Proper disposal and management of clozapine waste are essential to mitigate these effects.
Effects on Wildlife
Contaminants from clozapine can affect aquatic life and other wildlife. It is crucial to manage and dispose of clozapine waste properly to prevent environmental contamination.
Safety Measures
Strict monitoring and management protocols are in place to ensure the safe use of clozapine. These include regular blood tests, monitoring for signs of myocarditis, and managing side effects through dose adjustments and additional medications.
Regulation
Clozapine is regulated under various national and international guidelines. In the US, it is available only through a restricted program due to its severe side effects. Similar regulations exist in other countries to ensure patient safety.
Analytical Standards
HPC Standards GmbH provides high-purity reference materials for clozapine, ensuring accurate and reliable residue analysis. These reference materials meet international quality requirements and the highest industrial standards, essential for laboratories conducting food and environmental analysis to ensure compliance with regulatory limits.


