New user? / Forgot your password?
0 Item | 0,00 €
Flumequine Reference Materials
Ensure Compliance with High-Purity Flumequine Reference Materials
High-purity Flumequine reference materials are essential for precise and reliable residue analysis in food and environmental samples. As a synthetic chemotherapeutic antibiotic used in veterinary medicine, Flumequine is crucial for treating bacterial infections in livestock, ensuring their health and productivity. Regulatory bodies like the EMA and FDA have set maximum residue limits for Flumequine in food products, making compliance vital for market access and consumer safety. HPC Standards GmbH provides top-quality reference materials, tested to international standards, to support laboratories in achieving accurate and compliant results.
Product | Catalog No./ CAS No. | Quantity | Price | |
|---|---|---|---|---|
 | 679579 | 1X10MG | Please log in. | |
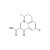 | 675462 | 1X250MG | Please log in. | |
Flumequine solution | 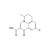 | 679797 | 1X1ML | Please log in. |
ISO 17034 Certified Reference Material | 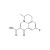 | 693529 | 1X100MG | Please log in. |
High-purity reference materials for precise and reliable residue analysis of Flumequine in food and environmental samples.
Overview
Flumequine is a synthetic chemotherapeutic antibiotic of the fluoroquinolone drug class. It is primarily used in veterinary medicine to treat bacterial infections in animals.
Uses
Flumequine is used to treat a variety of bacterial infections in livestock, including poultry, cattle, and fish. Its application ensures the health and productivity of these animals, which is critical for food production industries.
Regulatory
Regulatory bodies such as the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) have established maximum residue limits (MRLs) for Flumequine in food products derived from treated animals. Compliance with these limits is essential for market access and consumer safety.
Monitoring
Monitoring the residues of Flumequine in food and environmental samples is crucial for ensuring that they do not exceed regulatory limits. Laboratories use high-purity reference materials to calibrate their analytical instruments and validate their testing methods.
Health Impact
Human Toxicity
Exposure to Flumequine residues in food can pose health risks to humans, including potential allergic reactions and antimicrobial resistance. Thus, monitoring and controlling its levels in food products is vital.
Environmental Impact
Effects on Wildlife
Flumequine can enter the environment through agricultural runoff and improper disposal of veterinary products. It can affect aquatic ecosystems and wildlife, potentially disrupting local biodiversity.
Safety Measures
Proper handling and disposal of Flumequine and its residues are necessary to minimize environmental contamination. Laboratories and agricultural facilities must follow safety guidelines to protect both human health and the environment.
Regulation
Strict regulations govern the use of Flumequine in veterinary medicine. These include guidelines on dosage, administration, and withdrawal periods to ensure that residues in food products are within safe limits.
Analytical Standards
High-purity reference materials for Flumequine are essential for accurate residue analysis. HPC Standards GmbH provides these materials, tested according to international quality requirements, to support laboratories in achieving reliable and compliant results.


